FhuD: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
<table style="background-color:#ffffc0" cellpadding="8" width="95%" border="0"><tr><td>Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. [[User:Andrea Gorrell|Andrea Gorrell]].</td></tr> | <table style="background-color:#ffffc0" cellpadding="8" width="95%" border="0"><tr><td>Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. [[User:Andrea Gorrell|Andrea Gorrell]].</td></tr> | ||
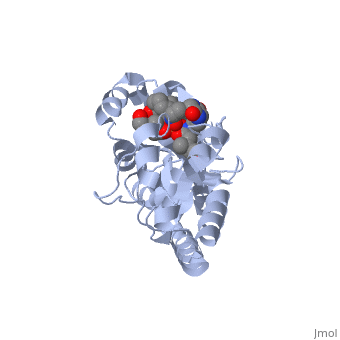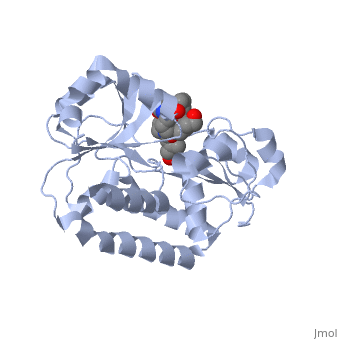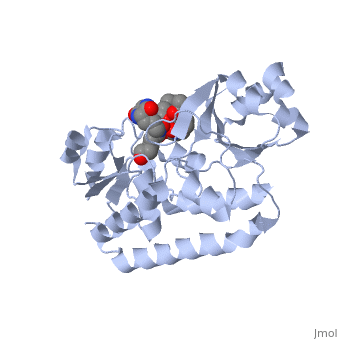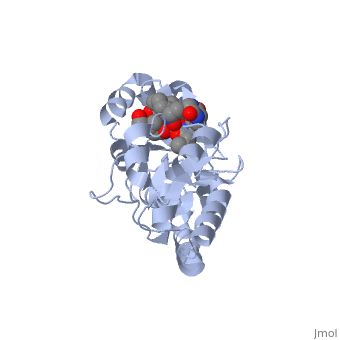
==PERIPLASMIC FERRIC SIDEROPHORE BINDING PROTEIN FHUD COMPLEXED WITH COPROGEN (1esz)== | =='''PERIPLASMIC FERRIC SIDEROPHORE BINDING PROTEIN FHUD COMPLEXED WITH COPROGEN (1esz)'''== | ||
<applet load='1esz' size='300' frame='true' align='right' caption='Insert caption here' /> | |||
==OVERVIEW== | ==OVERVIEW== | ||
Siderophore-binding proteins can be found in both Gram-positive and Gram-negative bacteria in two divisions: hydroxamates and catecholates. In Escherichia coli. (E. coli) the ATP-binding cassette- type (ABC-type) protein FhuD is a common periplasmic protein which facilitates the transport of a variety of hydoxamate siderophores to the inner membrane-associated proteins FhuB and FhuC. The structure of FhuD is atypical for periplasmic ligand binding protein due to its bilobal mixture of two α/β domains connected by long α-helix. | |||
==PROTEIN STRUCTURE== | |||
FhuD is atypical for periplasmic ligand binding proteins. It is a bilobal kidney bean shape containing two domains which are connected by a 23-residue kinked α-helix. The N-terminal domain twisted fived-stranded parallel β-sheet whereas the C-terminal domain has a mixed five stranded β-sheet; both are surrounded by α-helices. Between the two domains lies the siderophore binding in the shallow pocket. In this pocket, side chain residues are able to hydrogen bond with the accepted siderophore. | |||
==PROTEIN FUNCTION== | ==PROTEIN FUNCTION== | ||
[[User:Leni Rose|Leni Rose]] 04:57, 13 March 2010 (IST) | |||
Revision as of 05:57, 13 March 2010
PERIPLASMIC FERRIC SIDEROPHORE BINDING PROTEIN FHUD COMPLEXED WITH COPROGEN (1esz)PERIPLASMIC FERRIC SIDEROPHORE BINDING PROTEIN FHUD COMPLEXED WITH COPROGEN (1esz)
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |
|
OVERVIEWOVERVIEW
Siderophore-binding proteins can be found in both Gram-positive and Gram-negative bacteria in two divisions: hydroxamates and catecholates. In Escherichia coli. (E. coli) the ATP-binding cassette- type (ABC-type) protein FhuD is a common periplasmic protein which facilitates the transport of a variety of hydoxamate siderophores to the inner membrane-associated proteins FhuB and FhuC. The structure of FhuD is atypical for periplasmic ligand binding protein due to its bilobal mixture of two α/β domains connected by long α-helix.
PROTEIN STRUCTUREPROTEIN STRUCTURE
FhuD is atypical for periplasmic ligand binding proteins. It is a bilobal kidney bean shape containing two domains which are connected by a 23-residue kinked α-helix. The N-terminal domain twisted fived-stranded parallel β-sheet whereas the C-terminal domain has a mixed five stranded β-sheet; both are surrounded by α-helices. Between the two domains lies the siderophore binding in the shallow pocket. In this pocket, side chain residues are able to hydrogen bond with the accepted siderophore.
PROTEIN FUNCTIONPROTEIN FUNCTION
Leni Rose 04:57, 13 March 2010 (IST)