Triose Phosphate Isomerase Structure & Mechanism: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
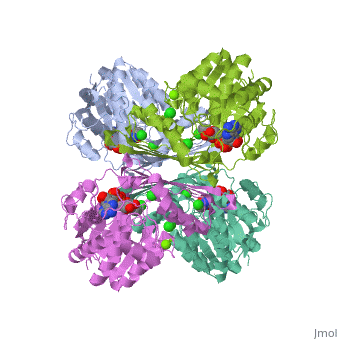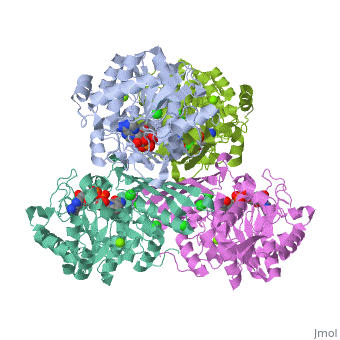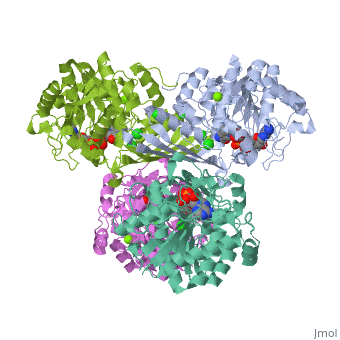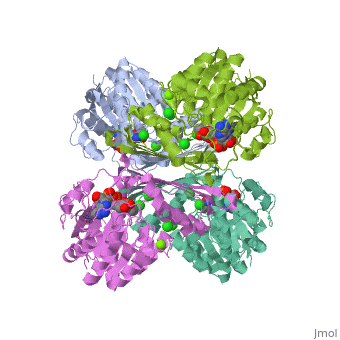
Triose phosphate isomerase (TIM) is a crucial enzyme in the glycolytic pathway. TIM reversibly converts the aldose Glyceraldehyde-3-phosphate (GASP) to the ketose Dihydroxyacetone phosphate (DHAP). The interconversion proceeds by an enediol intermediate. | Triose phosphate isomerase (TIM) is a crucial enzyme in the glycolytic pathway. TIM reversibly converts the aldose Glyceraldehyde-3-phosphate (GASP) to the ketose Dihydroxyacetone phosphate (DHAP). The interconversion proceeds by an enediol intermediate. | ||
==Mechanism of TIM== | |||
The enzyme aids in catalysis by binding tightly to the enediol transition state. To convert GAP to the enediol intermediate, a proton is abstracted from C2 by a base and the carbonyl oxygen atom is protonated by an acid. TIM’s Glu 165 acts as the base and grabs GAP’s C2 proton, while His 95 is H-bonded to the carbonyl oxygen and acts as the acid by protonating carbonyl oxygen. The enediol intermediate is negatively charged, but is somewhat stabilized by Lys 12’s positively charged side chain. To convert the enediol intermediate to DHAP, C1 is protonated by Glu 165, with His 95 removing a proton from C2’s OH group. As a result, the catalytic groups are back at their original states, and catalysis is completed. | |||
Replace the PDB id (use lowercase!) after the STRUCTURE_ and after PDB= to load | Replace the PDB id (use lowercase!) after the STRUCTURE_ and after PDB= to load | ||
Revision as of 16:16, 1 March 2010
Triose Phosphate Isomerase (TIM)Triose Phosphate Isomerase (TIM)
This is a placeholder text to help you get started in placing a Jmol applet on your page. At any time, click "Show Preview" at the bottom of this page to see how it goes.
Triose phosphate isomerase (TIM) is a crucial enzyme in the glycolytic pathway. TIM reversibly converts the aldose Glyceraldehyde-3-phosphate (GASP) to the ketose Dihydroxyacetone phosphate (DHAP). The interconversion proceeds by an enediol intermediate.
Mechanism of TIMMechanism of TIM
The enzyme aids in catalysis by binding tightly to the enediol transition state. To convert GAP to the enediol intermediate, a proton is abstracted from C2 by a base and the carbonyl oxygen atom is protonated by an acid. TIM’s Glu 165 acts as the base and grabs GAP’s C2 proton, while His 95 is H-bonded to the carbonyl oxygen and acts as the acid by protonating carbonyl oxygen. The enediol intermediate is negatively charged, but is somewhat stabilized by Lys 12’s positively charged side chain. To convert the enediol intermediate to DHAP, C1 is protonated by Glu 165, with His 95 removing a proton from C2’s OH group. As a result, the catalytic groups are back at their original states, and catalysis is completed.
Replace the PDB id (use lowercase!) after the STRUCTURE_ and after PDB= to load and display another structure.
| |||||||||
| 3cin, resolution 1.70Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Gene: | TM1419, TM_1419 (Thermotoga maritima MSB8) | ||||||||
| Activity: | Inositol-3-phosphate synthase, with EC number 5.5.1.4 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum, TOPSAN | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||