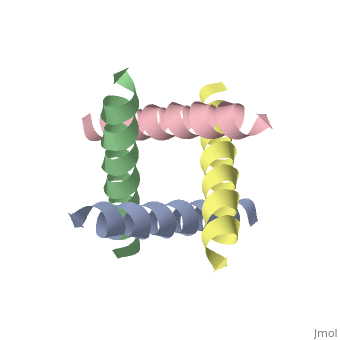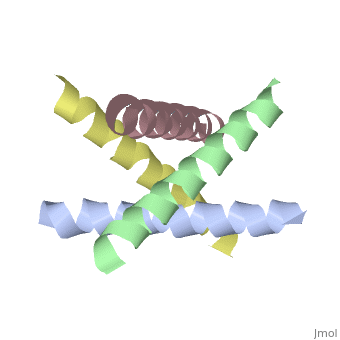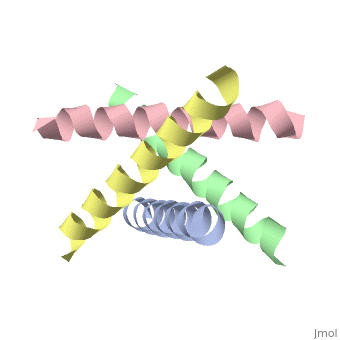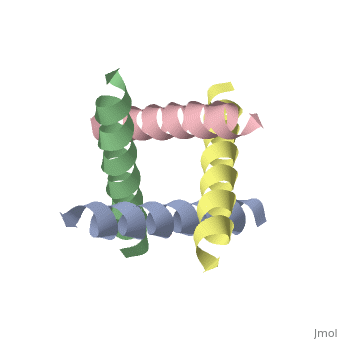M2 Proton Channel: Difference between revisions
Sarah Henke (talk | contribs) No edit summary |
Sarah Henke (talk | contribs) No edit summary |
||
| Line 3: | Line 3: | ||
== Background == | == Background == | ||
The M2 proton channel is a key protein that leads to viral infection [Takeuchi et al]. The M2 proton channel acidifies the viron which allows the viral matrix protein (M1) to disassociate from the ribonucleoprotein (RNP) [wu et al]. This allows the RNP to be transported to the nucleus of the cell [wu et al]. Several recent studies have looked at the effects of <scene name='User:Sarah_Henke/Sandbox_1/Amantadine/1'>amantadine</scene> <ref>PMID:12403618</ref> and <scene name='User:Sarah_Henke/Sandbox_1/Rimantadine/1'>rimantadine</scene> [Schnell et al] on inhibiting the transfer of protons through the M2 channel <ref | The M2 proton channel is a key protein that leads to viral infection [Takeuchi et al]. The M2 proton channel acidifies the viron which allows the viral matrix protein (M1) to disassociate from the ribonucleoprotein (RNP) [wu et al]. This allows the RNP to be transported to the nucleus of the cell [wu et al]. Several recent studies have looked at the effects of <scene name='User:Sarah_Henke/Sandbox_1/Amantadine/1'>amantadine</scene> <ref>PMID:12403618</ref> and <scene name='User:Sarah_Henke/Sandbox_1/Rimantadine/1'>rimantadine</scene> [Schnell et al] on inhibiting the transfer of protons through the M2 channel <ref name="Stouffer" />. It has been found that M2 is resistant to these two drugs in 90% of humans, birds and pigs <ref>PMID:12403618</ref>. Understanding the structure and function of this proton channel is necessary in solving the resistance problem <ref name="Stouffer" />. | ||
== Structure == | == Structure == | ||
The M2 proton channel from influenza A is 97 amino acid residues and forms a 24-residue N-terminal extracellular domain, a 19-residue trans-membrane domain, and a 54-residue C-terminal cytoplasmic domain [wu et al]. The 19-residue TM domain forms the highly selective proton channel [Takashi et al]. Circular dichroism spectra has shown the TM domain to form one α-helix that spans the membrane [wu et al]. By analytical ultracentrifugation, the TM domain is found to form <scene name='User:Sarah_Henke/Sandbox_1/Alpha_hlix/1'>α-helical tetramers</scene>[takeuchi et al]. This tetrameric bundle of the TM domain is found by NMR to be tilted by 25-38° from the channel axis [takeuchi et al]. The trameric helices form a left-handed bundle that resembles a truncated cone <ref | The M2 proton channel from influenza A is 97 amino acid residues and forms a 24-residue N-terminal extracellular domain, a 19-residue trans-membrane domain, and a 54-residue C-terminal cytoplasmic domain [wu et al]. The 19-residue TM domain forms the highly selective proton channel [Takashi et al]. Circular dichroism spectra has shown the TM domain to form one α-helix that spans the membrane [wu et al]. By analytical ultracentrifugation, the TM domain is found to form <scene name='User:Sarah_Henke/Sandbox_1/Alpha_hlix/1'>α-helical tetramers</scene>[takeuchi et al]. This tetrameric bundle of the TM domain is found by NMR to be tilted by 25-38° from the channel axis [takeuchi et al]. The trameric helices form a left-handed bundle that resembles a truncated cone <ref name="Stouffer" />. The TM helicies are arranged around the channel pore with an approximate fourfold rotational symmetry [takeuchi et al]. | ||
== Central Cavity == | == Central Cavity == | ||
Revision as of 06:41, 30 September 2009
M2 Proton Channel from Influenza A VirusM2 Proton Channel from Influenza A Virus
|
BackgroundBackground
The M2 proton channel is a key protein that leads to viral infection [Takeuchi et al]. The M2 proton channel acidifies the viron which allows the viral matrix protein (M1) to disassociate from the ribonucleoprotein (RNP) [wu et al]. This allows the RNP to be transported to the nucleus of the cell [wu et al]. Several recent studies have looked at the effects of [1] and [Schnell et al] on inhibiting the transfer of protons through the M2 channel [2]. It has been found that M2 is resistant to these two drugs in 90% of humans, birds and pigs [3]. Understanding the structure and function of this proton channel is necessary in solving the resistance problem [2].
StructureStructure
The M2 proton channel from influenza A is 97 amino acid residues and forms a 24-residue N-terminal extracellular domain, a 19-residue trans-membrane domain, and a 54-residue C-terminal cytoplasmic domain [wu et al]. The 19-residue TM domain forms the highly selective proton channel [Takashi et al]. Circular dichroism spectra has shown the TM domain to form one α-helix that spans the membrane [wu et al]. By analytical ultracentrifugation, the TM domain is found to form [takeuchi et al]. This tetrameric bundle of the TM domain is found by NMR to be tilted by 25-38° from the channel axis [takeuchi et al]. The trameric helices form a left-handed bundle that resembles a truncated cone [2]. The TM helicies are arranged around the channel pore with an approximate fourfold rotational symmetry [takeuchi et al].
Central CavityCentral Cavity
|
pH GatingpH Gating
ReferencesReferences
- ↑ Nishimura K, Kim S, Zhang L, Cross TA. The closed state of a H+ channel helical bundle combining precise orientational and distance restraints from solid state NMR. Biochemistry. 2002 Nov 5;41(44):13170-7. PMID:12403618
- ↑ 2.0 2.1 2.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedStouffer - ↑ Nishimura K, Kim S, Zhang L, Cross TA. The closed state of a H+ channel helical bundle combining precise orientational and distance restraints from solid state NMR. Biochemistry. 2002 Nov 5;41(44):13170-7. PMID:12403618