1st6: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
==Crystal structure of a cytoskeletal protein== | ==Crystal structure of a cytoskeletal protein== | ||
<StructureSection load='1st6' size='340' side='right' caption='[[1st6]], [[Resolution|resolution]] 3.10Å' scene=''> | <StructureSection load='1st6' size='340' side='right'caption='[[1st6]], [[Resolution|resolution]] 3.10Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1st6]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Chick Chick]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1ST6 OCA]. For a <b>guided tour on the structure components</b> use [http:// | <table><tr><td colspan='2'>[[1st6]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Chick Chick]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1ST6 OCA]. For a <b>guided tour on the structure components</b> use [http://proteopedia.org/fgij/fg.htm?mol=1ST6 FirstGlance]. <br> | ||
</td></tr><tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">VCL ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9031 CHICK])</td></tr> | </td></tr><tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">VCL ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9031 CHICK])</td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http:// | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://proteopedia.org/fgij/fg.htm?mol=1st6 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1st6 OCA], [http://pdbe.org/1st6 PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=1st6 RCSB], [http://www.ebi.ac.uk/pdbsum/1st6 PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=1st6 ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
| Line 36: | Line 36: | ||
</StructureSection> | </StructureSection> | ||
[[Category: Chick]] | [[Category: Chick]] | ||
[[Category: Large Structures]] | |||
[[Category: Bakolitsa, C]] | [[Category: Bakolitsa, C]] | ||
[[Category: Liddington, R C]] | [[Category: Liddington, R C]] | ||
[[Category: Cell adhesion]] | [[Category: Cell adhesion]] | ||
[[Category: Up-down bundle]] | [[Category: Up-down bundle]] | ||
Revision as of 14:33, 24 December 2020
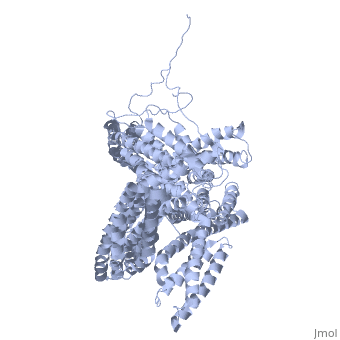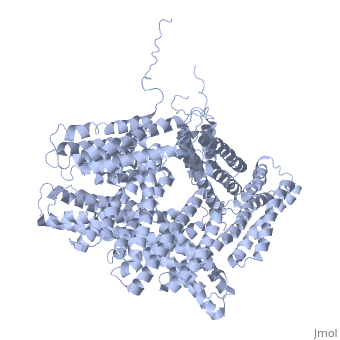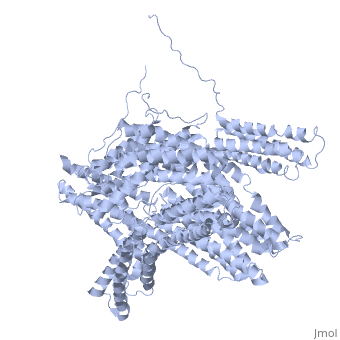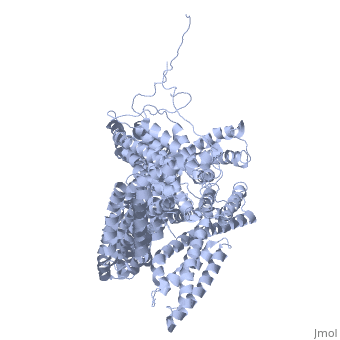
Crystal structure of a cytoskeletal proteinCrystal structure of a cytoskeletal protein
Structural highlights
Function[VINC_CHICK] Actin filament (F-actin)-binding protein involved in cell-matrix adhesion and cell-cell adhesion. Regulates cell-surface E-cadherin expression and potentiates mechanosensing by the E-cadherin complex. May also play important roles in cell morphology and locomotion.[1] [2] [3] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedVinculin is a highly conserved intracellular protein with a crucial role in the maintenance and regulation of cell adhesion and migration. In the cytosol, vinculin adopts a default autoinhibited conformation. On recruitment to cell-cell and cell-matrix adherens-type junctions, vinculin becomes activated and mediates various protein-protein interactions that regulate the links between F-actin and the cadherin and integrin families of cell-adhesion molecules. Here we describe the crystal structure of the full-length vinculin molecule (1,066 amino acids), which shows a five-domain autoinhibited conformation in which the carboxy-terminal tail domain is held pincer-like by the vinculin head, and ligand binding is regulated both sterically and allosterically. We show that conformational changes in the head, tail and proline-rich domains are linked structurally and thermodynamically, and propose a combinatorial pathway to activation that ensures that vinculin is activated only at sites of cell adhesion when two or more of its binding partners are brought into apposition. Structural basis for vinculin activation at sites of cell adhesion.,Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, Critchley DR, Craig SW, Liddington RC Nature. 2004 Jul 29;430(6999):583-6. Epub 2004 Jun 13. PMID:15195105[4] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||