1dq8: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:1dq8.gif|left|200px]] | [[Image:1dq8.gif|left|200px]] | ||
<!-- | |||
The line below this paragraph, containing "STRUCTURE_1dq8", creates the "Structure Box" on the page. | |||
You may change the PDB parameter (which sets the PDB file loaded into the applet) | |||
or the SCENE parameter (which sets the initial scene displayed when the page is loaded), | |||
or leave the SCENE parameter empty for the default display. | |||
| | --> | ||
| | {{STRUCTURE_1dq8| PDB=1dq8 | SCENE= }} | ||
}} | |||
'''COMPLEX OF THE CATALYTIC PORTION OF HUMAN HMG-COA REDUCTASE WITH HMG AND COA''' | '''COMPLEX OF THE CATALYTIC PORTION OF HUMAN HMG-COA REDUCTASE WITH HMG AND COA''' | ||
| Line 24: | Line 21: | ||
Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis., Istvan ES, Palnitkar M, Buchanan SK, Deisenhofer J, EMBO J. 2000 Mar 1;19(5):819-30. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10698924 10698924] | Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis., Istvan ES, Palnitkar M, Buchanan SK, Deisenhofer J, EMBO J. 2000 Mar 1;19(5):819-30. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10698924 10698924] | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
[[Category: Buchanan, S K.]] | [[Category: Buchanan, S K.]] | ||
| Line 30: | Line 26: | ||
[[Category: Istvan, E S.]] | [[Category: Istvan, E S.]] | ||
[[Category: Palnitkar, M.]] | [[Category: Palnitkar, M.]] | ||
[[Category: | [[Category: Cholesterol biosynthesis]] | ||
[[Category: | [[Category: Hmg-coa]] | ||
[[Category: | [[Category: Nadph]] | ||
[[Category: | [[Category: Oxidoreductase]] | ||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Fri May 2 14:08:44 2008'' | |||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | |||
Revision as of 14:08, 2 May 2008
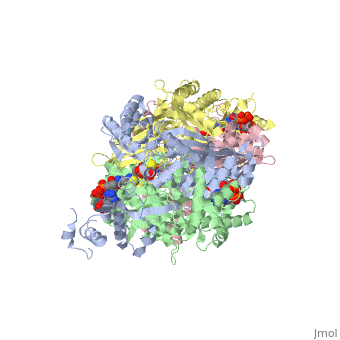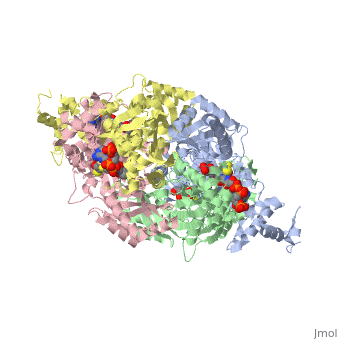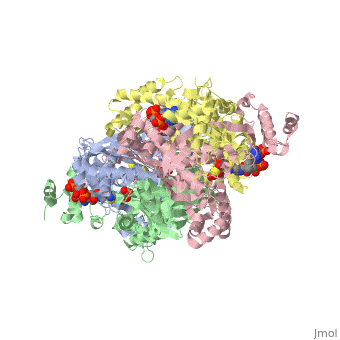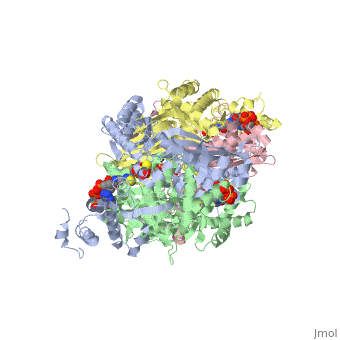
COMPLEX OF THE CATALYTIC PORTION OF HUMAN HMG-COA REDUCTASE WITH HMG AND COA
OverviewOverview
3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) catalyzes the formation of mevalonate, the committed step in the biosynthesis of sterols and isoprenoids. The activity of HMGR is controlled through synthesis, degradation and phosphorylation to maintain the concentration of mevalonate-derived products. In addition to the physiological regulation of HMGR, the human enzyme has been targeted successfully by drugs in the clinical treatment of high serum cholesterol levels. Three crystal structures of the catalytic portion of human HMGR in complexes with HMG-CoA, with HMG and CoA, and with HMG, CoA and NADP(+), provide a detailed view of the enzyme active site. Catalytic portions of human HMGR form tight tetramers. The crystal structure explains the influence of the enzyme's oligomeric state on the activity and suggests a mechanism for cholesterol sensing. The active site architecture of human HMGR is different from that of bacterial HMGR; this may explain why binding of HMGR inhibitors to bacterial HMGRs has not been reported.
About this StructureAbout this Structure
1DQ8 is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
ReferenceReference
Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis., Istvan ES, Palnitkar M, Buchanan SK, Deisenhofer J, EMBO J. 2000 Mar 1;19(5):819-30. PMID:10698924 Page seeded by OCA on Fri May 2 14:08:44 2008