1e6m: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1e6m]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1E6M OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1E6M FirstGlance]. <br> | <table><tr><td colspan='2'>[[1e6m]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1E6M OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1E6M FirstGlance]. <br> | ||
</td></tr><tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1udr|1udr]], [[1eay|1eay]], [[1c4w|1c4w]], [[1bdj|1bdj]], [[1ab5|1ab5]], [[1ab6|1ab6]], [[1a0o|1a0o]], [[1e6k|1e6k]], [[1e6l|1e6l]], [[3chy|3chy]], [[5chy|5chy]], [[6chy|6chy]], [[1chn|1chn]], [[1cey|1cey]], [[1ymu|1ymu]], [[1ymv|1ymv]], [[1cye|1cye]], [[1vlz|1vlz]], [[1ehc|1ehc]], [[1hey|1hey]], [[1d4z|1d4z]], [[1djm|1djm]]</td></tr> | </td></tr><tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1udr|1udr]], [[1eay|1eay]], [[1c4w|1c4w]], [[1bdj|1bdj]], [[1ab5|1ab5]], [[1ab6|1ab6]], [[1a0o|1a0o]], [[1e6k|1e6k]], [[1e6l|1e6l]], [[3chy|3chy]], [[5chy|5chy]], [[6chy|6chy]], [[1chn|1chn]], [[1cey|1cey]], [[1ymu|1ymu]], [[1ymv|1ymv]], [[1cye|1cye]], [[1vlz|1vlz]], [[1ehc|1ehc]], [[1hey|1hey]], [[1d4z|1d4z]], [[1djm|1djm]]</td></tr> | ||
<tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1e6m FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1e6m OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1e6m RCSB], [http://www.ebi.ac.uk/pdbsum/1e6m PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1e6m FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1e6m OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1e6m RCSB], [http://www.ebi.ac.uk/pdbsum/1e6m PDBsum]</span></td></tr> | ||
<table> | </table> | ||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 32: | Line 32: | ||
</StructureSection> | </StructureSection> | ||
[[Category: Escherichia coli]] | [[Category: Escherichia coli]] | ||
[[Category: Coll, M | [[Category: Coll, M]] | ||
[[Category: Cronet, P | [[Category: Cronet, P]] | ||
[[Category: Lacroix, E | [[Category: Lacroix, E]] | ||
[[Category: Lopez-Hernandez, E | [[Category: Lopez-Hernandez, E]] | ||
[[Category: Parraga, A | [[Category: Parraga, A]] | ||
[[Category: Serrano, L | [[Category: Serrano, L]] | ||
[[Category: Sola, M | [[Category: Sola, M]] | ||
[[Category: Active site mutant]] | [[Category: Active site mutant]] | ||
[[Category: Chemotaxis]] | [[Category: Chemotaxis]] | ||
[[Category: Signaling protein]] | [[Category: Signaling protein]] | ||
[[Category: Two-component signal transduction system]] | [[Category: Two-component signal transduction system]] | ||
Revision as of 02:27, 23 December 2014
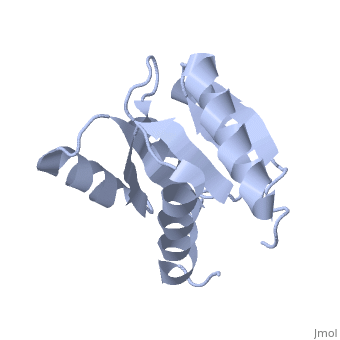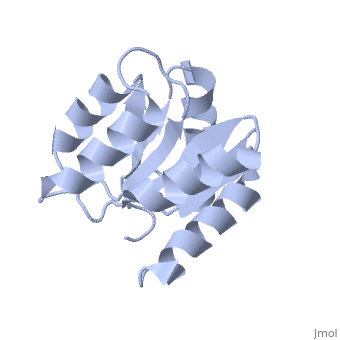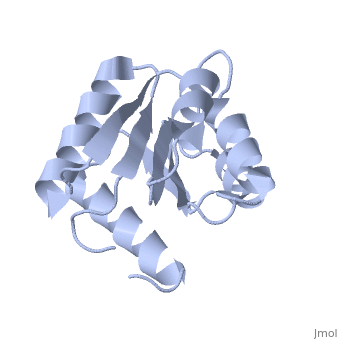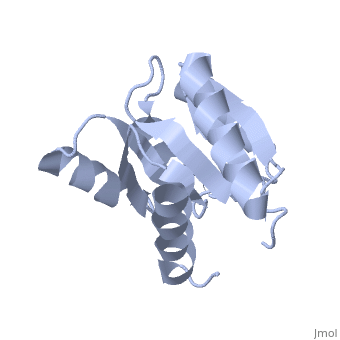
D57A mutant of CheYD57A mutant of CheY
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe signal transduction protein CheY displays an alpha/beta-parallel polypeptide folding, including a highly unstable helix alpha4 and a strongly charged active site. Helix alpha4 has been shown to adopt various positions and conformations in different crystal structures, suggesting that it is a mobile segment. Furthermore, the instability of this helix is believed to have functional significance because it is involved in protein-protein contacts with the transmitter protein kinase CheA, the target protein FliM and the phosphatase CheZ. The active site of CheY comprises a cluster of three aspartic acid residues and a lysine residue, all of which participate in the binding of the Mg(2+) needed for the protein activation. Two steps were followed to study the activation mechanism of CheY upon phosphorylation: first, we independently substituted the three aspartic acid residues in the active site with alanine; second, several mutations were designed in helix alpha 4, both to increase its level of stability and to improve its packing against the protein core. The structural and thermodynamic analysis of these mutant proteins provides further evidence of the connection between the active-site area and helix alpha 4, and helps to understand how small movements at the active site are transmitted and amplified to the protein surface. Towards understanding a molecular switch mechanism: thermodynamic and crystallographic studies of the signal transduction protein CheY.,Sola M, Lopez-Hernandez E, Cronet P, Lacroix E, Serrano L, Coll M, Parraga A J Mol Biol. 2000 Oct 20;303(2):213-25. PMID:11023787[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||