Ann Taylor sandbox 1: Difference between revisions
No edit summary |
Ann Taylor (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{Gal4 practice page}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
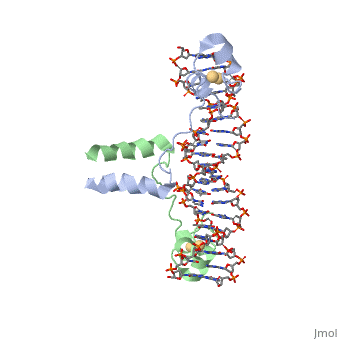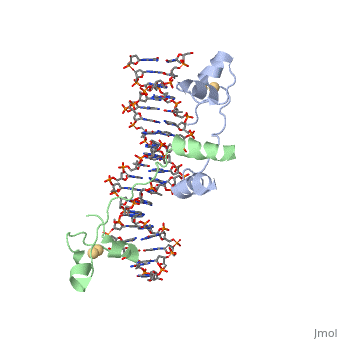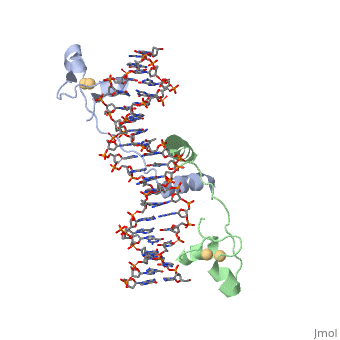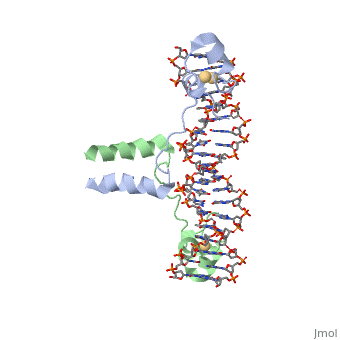
==DNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEX== | |||
<StructureSection load='1d66' size='340' side='right'caption='[[1d66]], [[Resolution|resolution]] 2.70Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1d66]] is a 4 chain structure with sequence from [https://en.wikipedia.org/wiki/Atcc_18824 Atcc 18824]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1D66 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1D66 FirstGlance]. <br> | |||
</td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=CD:CADMIUM+ION'>CD</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1d66 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1d66 OCA], [https://pdbe.org/1d66 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1d66 RCSB], [https://www.ebi.ac.uk/pdbsum/1d66 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1d66 ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[[https://www.uniprot.org/uniprot/GAL4_YEAST GAL4_YEAST]] This protein is a positive regulator for the gene expression of the galactose-induced genes such as GAL1, GAL2, GAL7, GAL10, and MEL1 which code for the enzymes used to convert galactose to glucose. It recognizes a 17 base pair sequence in (5'-CGGRNNRCYNYNCNCCG-3') the upstream activating sequence (UAS-G) of these genes. | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/d6/1d66_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1d66 ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
A specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a dimer to a symmetrical 17-base-pair sequence. A small, Zn(2+)-containing domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4. | |||
Gal4 has lots of <scene name='89/891376/Basic_aas/2'>basic amino acids</scene> that interact with DNA. | |||
DNA recognition by GAL4: structure of a protein-DNA complex.,Marmorstein R, Carey M, Ptashne M, Harrison SC Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122<ref>PMID:1557122</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 1d66" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Gal3-Gal80-Gal4|Gal3-Gal80-Gal4]] | |||
*[[Hydrogen in macromolecular models|Hydrogen in macromolecular models]] | |||
== References == | |||
<references/> | <references/> | ||
__TOC__ | |||
</StructureSection> | |||
[[Category: Atcc 18824]] | |||
[[Category: Large Structures]] | |||
[[Category: Carey, M]] | |||
[[Category: Harrison, S C]] | |||
[[Category: Marmorstein, R]] | |||
[[Category: Ptashne, M]] | |||
[[Category: Double helix]] | |||
[[Category: Protein-dna complex]] | |||
[[Category: Transcription-dna complex]] | |||
Revision as of 15:55, 7 September 2023
| This Sandbox is Reserved from 09/01/2021 through 12/01/2021 for use in Che 462 taught by Ann Taylor at Wabash College, Crawfordsville, IN USA. This reservation includes Sandbox Reserved 1683 through Sandbox Reserved 1689. |
To get started:
More help: Help:Editing |
DNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEXDNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEX
Structural highlights
Function[GAL4_YEAST] This protein is a positive regulator for the gene expression of the galactose-induced genes such as GAL1, GAL2, GAL7, GAL10, and MEL1 which code for the enzymes used to convert galactose to glucose. It recognizes a 17 base pair sequence in (5'-CGGRNNRCYNYNCNCCG-3') the upstream activating sequence (UAS-G) of these genes. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedA specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a dimer to a symmetrical 17-base-pair sequence. A small, Zn(2+)-containing domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4. Gal4 has lots of that interact with DNA. DNA recognition by GAL4: structure of a protein-DNA complex.,Marmorstein R, Carey M, Ptashne M, Harrison SC Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||