4ald: Difference between revisions
New page: left|200px<br /> <applet load="4ald" size="450" color="white" frame="true" align="right" spinBox="true" caption="4ald, resolution 2.8Å" /> '''HUMAN MUSCLE FRUCTOS... |
No edit summary |
||
| Line 8: | Line 8: | ||
==About this Structure== | ==About this Structure== | ||
4ALD is a [[http://en.wikipedia.org/wiki/Single_protein Single protein]] structure of sequence from [[http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]] with 2FP as [[http://en.wikipedia.org/wiki/ligand ligand]]. The following page contains interesting information on the relation of 4ALD with [[http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb50_1.html The Glycolytic Enzymes]]. Active as [[http://en.wikipedia.org/wiki/ ]], with EC number [[http://www.brenda-enzymes.info/php/result_flat.php4?ecno=4.1.2.13 4.1.2.13]]. Full crystallographic information is available from [[http://ispc.weizmann.ac.il/oca-bin/ocashort?id=4ALD OCA]]. | 4ALD is a [[http://en.wikipedia.org/wiki/Single_protein Single protein]] structure of sequence from [[http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]] with 2FP as [[http://en.wikipedia.org/wiki/ligand ligand]]. The following page contains interesting information on the relation of 4ALD with [[http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb50_1.html The Glycolytic Enzymes]]. Active as [[http://en.wikipedia.org/wiki/Lyase Lyase]], with EC number [[http://www.brenda-enzymes.info/php/result_flat.php4?ecno=4.1.2.13 4.1.2.13]]. Structure known Active Site: SBL. Full crystallographic information is available from [[http://ispc.weizmann.ac.il/oca-bin/ocashort?id=4ALD OCA]]. | ||
==Reference== | ==Reference== | ||
| Line 24: | Line 24: | ||
[[Category: type 1 aldolase]] | [[Category: type 1 aldolase]] | ||
''Page seeded by [http://ispc.weizmann.ac.il/oca OCA ] on | ''Page seeded by [http://ispc.weizmann.ac.il/oca OCA ] on Tue Oct 30 08:36:53 2007'' | ||
Revision as of 09:32, 30 October 2007
|
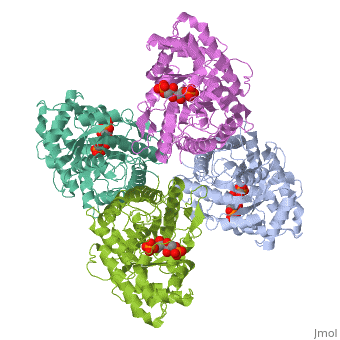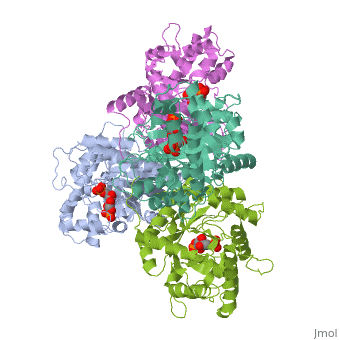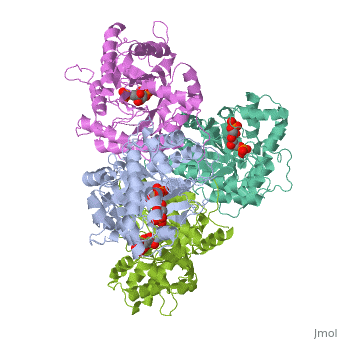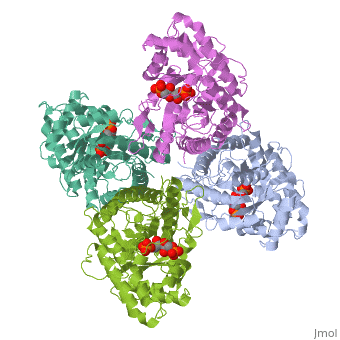
HUMAN MUSCLE FRUCTOSE 1,6-BISPHOSPHATE ALDOLASE COMPLEXED WITH FRUCTOSE 1,6-BISPHOSPHATE
OverviewOverview
Fructose 1,6-bisphosphate aldolase catalyzes the reversible cleavage of, fructose 1,6-bisphosphate and fructose 1-phosphate to dihydroxyacetone, phosphate and either glyceraldehyde 3-phosphate or glyceraldehyde, respectively. Catalysis involves the formation of a Schiff's base, intermediate formed at the epsilon-amino group of Lys229. The existing, apo-enzyme structure was refined using the crystallographic free-R-factor, and maximum likelihood methods that have been shown to give improved, structural results that are less subject to model bias. Crystals were also, soaked with the natural substrate (fructose 1,6-bisphosphate), and the, crystal structure of this complex has been determined to 2.8 A. The apo, structure differs from the previous Brookhaven-deposited structure (1ald), in the ... [(full description)]
About this StructureAbout this Structure
4ALD is a [Single protein] structure of sequence from [Homo sapiens] with 2FP as [ligand]. The following page contains interesting information on the relation of 4ALD with [The Glycolytic Enzymes]. Active as [Lyase], with EC number [4.1.2.13]. Structure known Active Site: SBL. Full crystallographic information is available from [OCA].
ReferenceReference
Crystal structure of human muscle aldolase complexed with fructose 1,6-bisphosphate: mechanistic implications., Dalby A, Dauter Z, Littlechild JA, Protein Sci. 1999 Feb;8(2):291-7. PMID:10048322
Page seeded by OCA on Tue Oct 30 08:36:53 2007