2abx: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:2abx.gif|left|200px]] | [[Image:2abx.gif|left|200px]] | ||
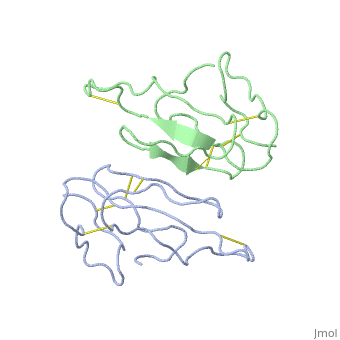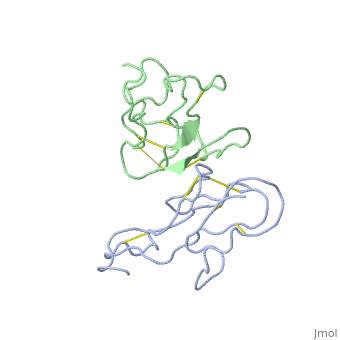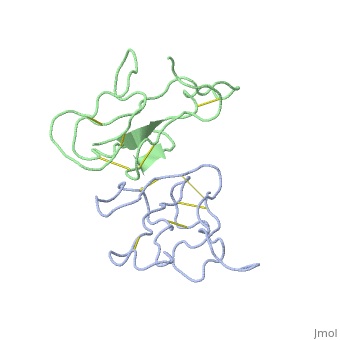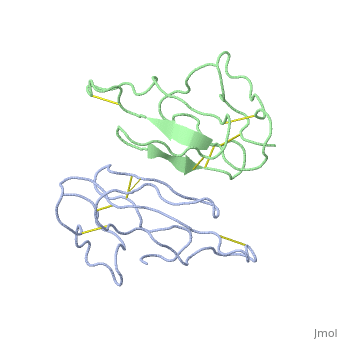
'''THE CRYSTAL STRUCTURE OF ALPHA-BUNGAROTOXIN AT 2.5 ANGSTROMS RESOLUTION. RELATION TO SOLUTION STRUCTURE AND BINDING TO ACETYLCHOLINE RECEPTOR''' | {{Structure | ||
|PDB= 2abx |SIZE=350|CAPTION= <scene name='initialview01'>2abx</scene>, resolution 2.5Å | |||
|SITE= | |||
|LIGAND= | |||
|ACTIVITY= | |||
|GENE= | |||
}} | |||
'''THE CRYSTAL STRUCTURE OF ALPHA-BUNGAROTOXIN AT 2.5 ANGSTROMS RESOLUTION. RELATION TO SOLUTION STRUCTURE AND BINDING TO ACETYLCHOLINE RECEPTOR''' | |||
==Overview== | ==Overview== | ||
| Line 7: | Line 16: | ||
==About this Structure== | ==About this Structure== | ||
2ABX is a [ | 2ABX is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Bungarus_multicinctus Bungarus multicinctus]. This structure supersedes the now removed PDB entry 1ABX. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2ABX OCA]. | ||
==Reference== | ==Reference== | ||
The crystal structure of alpha-bungarotoxin at 2.5 A resolution: relation to solution structure and binding to acetylcholine receptor., Love RA, Stroud RM, Protein Eng. 1986 Oct-Nov;1(1):37-46. PMID:[http:// | The crystal structure of alpha-bungarotoxin at 2.5 A resolution: relation to solution structure and binding to acetylcholine receptor., Love RA, Stroud RM, Protein Eng. 1986 Oct-Nov;1(1):37-46. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/3507686 3507686] | ||
[[Category: Bungarus multicinctus]] | [[Category: Bungarus multicinctus]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| Line 17: | Line 26: | ||
[[Category: postsynaptic neurotoxin]] | [[Category: postsynaptic neurotoxin]] | ||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 15:47:49 2008'' | ||
Revision as of 16:47, 20 March 2008
| |||||||
| , resolution 2.5Å | |||||||
|---|---|---|---|---|---|---|---|
| Coordinates: | save as pdb, mmCIF, xml | ||||||
THE CRYSTAL STRUCTURE OF ALPHA-BUNGAROTOXIN AT 2.5 ANGSTROMS RESOLUTION. RELATION TO SOLUTION STRUCTURE AND BINDING TO ACETYLCHOLINE RECEPTOR
OverviewOverview
We report collection of 2.5 A resolution X-ray diffraction data from newly grown crystals of the rare 'small unit cell' form of the long snake neurotoxin, alpha-bungarotoxin. The previous model of the molecule has been rebuilt, and refined using least-square methods to a crystallographic residual of 0.24 at 2.5 A resolution. alpha-Bungarotoxin's crystal structure is compared with the crystal structures of two other snake neurotoxins (cobratoxin and erabutoxin), and with its solution structure inferred from spectroscopic studies. Significant differences include less beta-sheet in bungarotoxin's crystal structure than in solution, or in the crystal structures of other neurotoxins, and an unusual orientation in the crystal of the invariant tryptophan. The functional, binding surface of bungarotoxin is described; it consists primarily of hydrophobic and hydrogen-bonding groups and only a few charged side-chains. The structure is compared with experimental binding parameters for neurotoxins.
About this StructureAbout this Structure
2ABX is a Single protein structure of sequence from Bungarus multicinctus. This structure supersedes the now removed PDB entry 1ABX. Full crystallographic information is available from OCA.
ReferenceReference
The crystal structure of alpha-bungarotoxin at 2.5 A resolution: relation to solution structure and binding to acetylcholine receptor., Love RA, Stroud RM, Protein Eng. 1986 Oct-Nov;1(1):37-46. PMID:3507686
Page seeded by OCA on Thu Mar 20 15:47:49 2008