1fv2: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
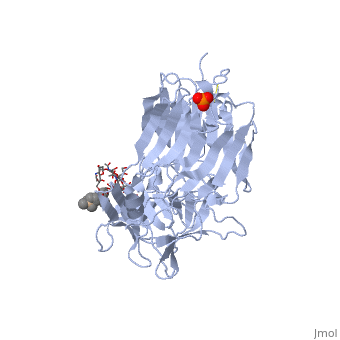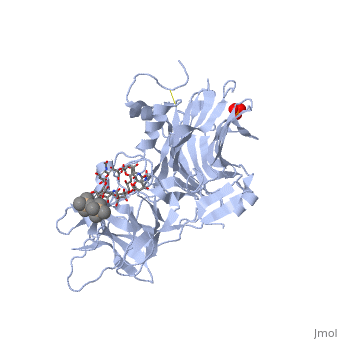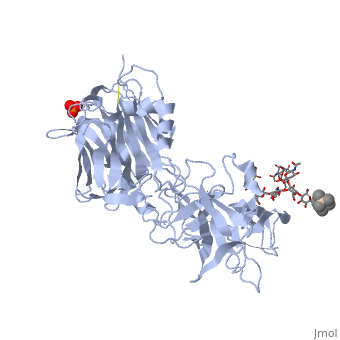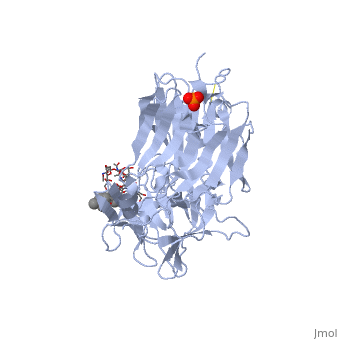
[[Image: | ==The Hc fragment of tetanus toxin complexed with an analogue of its ganglioside receptor GT1B== | ||
<StructureSection load='1fv2' size='340' side='right' caption='[[1fv2]], [[Resolution|resolution]] 2.50Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1fv2]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Clostridium_tetani Clostridium tetani]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1FV2 OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1FV2 FirstGlance]. <br> | |||
</td></tr><tr><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=BGC:BETA-D-GLUCOSE'>BGC</scene>, <scene name='pdbligand=CEQ:ETHYL-TRIMETHYL-SILANE'>CEQ</scene>, <scene name='pdbligand=PO4:PHOSPHATE+ION'>PO4</scene>, <scene name='pdbligand=GAL:BETA-D-GALACTOSE'>GAL</scene>, <scene name='pdbligand=NGA:N-ACETYL-D-GALACTOSAMINE'>NGA</scene><br> | |||
<tr><td class="sblockLbl"><b>[[Non-Standard_Residue|NonStd Res:]]</b></td><td class="sblockDat"><scene name='pdbligand=SIA:O-SIALIC+ACID'>SIA</scene>, <scene name='pdbligand=SLB:5-N-ACETYL-BETA-D-NEURAMINIC+ACID'>SLB</scene></td></tr> | |||
<tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1d0h|1d0h]], [[1dll|1dll]], [[1diw|1diw]], [[1dfq|1dfq]], [[1af9|1af9]], [[1a8d|1a8d]], [[1fv3|1fv3]]</td></tr> | |||
<tr><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Tentoxilysin Tentoxilysin], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.4.24.68 3.4.24.68] </span></td></tr> | |||
<tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1fv2 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1fv2 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1fv2 RCSB], [http://www.ebi.ac.uk/pdbsum/1fv2 PDBsum]</span></td></tr> | |||
<table> | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/fv/1fv2_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Tetanus toxin, a member of the family of Clostridial neurotoxins, is one of the most potent toxins known. The crystal structure of the complex of the COOH-terminal fragment of the heavy chain with an analogue of its ganglioside receptor, GT1b, provides the first direct identification and characterization of the ganglioside-binding sites. The ganglioside induces cross-linking by binding to two distinct sites on the Hc molecule. The structure sheds new light on the binding of Clostridial neurotoxins to receptors on neuronal cells and provides important information relevant to the design of anti-tetanus and anti-botulism therapeutic agents. | |||
The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin.,Fotinou C, Emsley P, Black I, Ando H, Ishida H, Kiso M, Sinha KA, Fairweather NF, Isaacs NW J Biol Chem. 2001 Aug 24;276(34):32274-81. Epub 2001 Jun 19. PMID:11418600<ref>PMID:11418600</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
==See Also== | ==See Also== | ||
*[[Tetanus toxin|Tetanus toxin]] | *[[Tetanus toxin|Tetanus toxin]] | ||
== References == | |||
== | <references/> | ||
< | __TOC__ | ||
</StructureSection> | |||
[[Category: Clostridium tetani]] | [[Category: Clostridium tetani]] | ||
[[Category: Tentoxilysin]] | [[Category: Tentoxilysin]] | ||
Revision as of 16:37, 28 September 2014
The Hc fragment of tetanus toxin complexed with an analogue of its ganglioside receptor GT1BThe Hc fragment of tetanus toxin complexed with an analogue of its ganglioside receptor GT1B
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedTetanus toxin, a member of the family of Clostridial neurotoxins, is one of the most potent toxins known. The crystal structure of the complex of the COOH-terminal fragment of the heavy chain with an analogue of its ganglioside receptor, GT1b, provides the first direct identification and characterization of the ganglioside-binding sites. The ganglioside induces cross-linking by binding to two distinct sites on the Hc molecule. The structure sheds new light on the binding of Clostridial neurotoxins to receptors on neuronal cells and provides important information relevant to the design of anti-tetanus and anti-botulism therapeutic agents. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin.,Fotinou C, Emsley P, Black I, Ando H, Ishida H, Kiso M, Sinha KA, Fairweather NF, Isaacs NW J Biol Chem. 2001 Aug 24;276(34):32274-81. Epub 2001 Jun 19. PMID:11418600[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||||||