2d1w: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image: | ==Substrate Schiff-Base intermediate with tyramine in copper amine oxidase from Arthrobacter globiformis== | ||
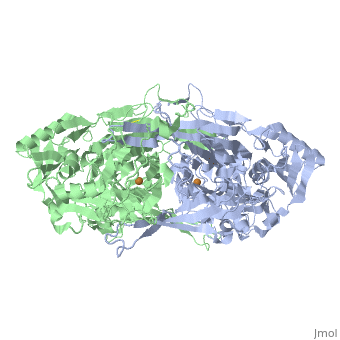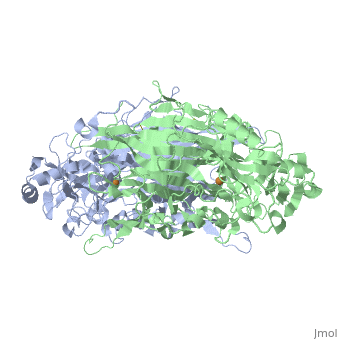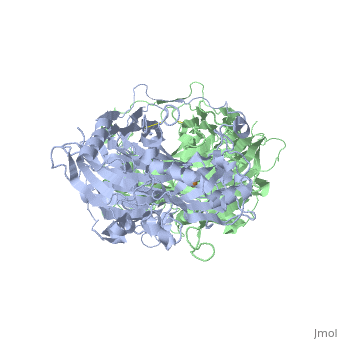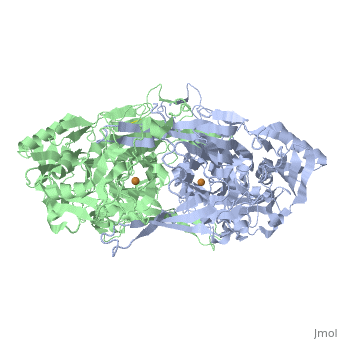
<StructureSection load='2d1w' size='340' side='right' caption='[[2d1w]], [[Resolution|resolution]] 1.74Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[2d1w]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Arthrobacter_globiformis Arthrobacter globiformis]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2D1W OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2D1W FirstGlance]. <br> | |||
</td></tr><tr><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=CU:COPPER+(II)+ION'>CU</scene><br> | |||
<tr><td class="sblockLbl"><b>[[Non-Standard_Residue|NonStd Res:]]</b></td><td class="sblockDat"><scene name='pdbligand=TTS:3-((3E)-4-HYDROXY-3-{[2-(4-HYDROXYPHENYL)ETHYL]IMINO}-6-OXOCYCLOHEXA-1,4-DIEN-1-YL)ALANINE'>TTS</scene></td></tr> | |||
<tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1iu7|1iu7]], [[2cwt|2cwt]], [[2cwu|2cwu]], [[2cwv|2cwv]]</td></tr> | |||
<tr><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Oxidoreductase Oxidoreductase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=1.4.3.21 and 1.4.3.22 1.4.3.21 and 1.4.3.22] </span></td></tr> | |||
<tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2d1w FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2d1w OCA], [http://www.rcsb.org/pdb/explore.do?structureId=2d1w RCSB], [http://www.ebi.ac.uk/pdbsum/2d1w PDBsum]</span></td></tr> | |||
<table> | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/d1/2d1w_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
A key step decisively affecting the catalytic efficiency of copper amine oxidase is stereospecific abstraction of substrate alpha-proton by a conserved Asp residue. We analyzed this step by pre-steady-state kinetics using a bacterial enzyme and stereospecifically deuterium-labeled substrates, 2-phenylethylamine and tyramine. A small and temperature-dependent kinetic isotope effect (KIE) was observed with 2-phenylethylamine, whereas a large and temperature-independent KIE was observed with tyramine in the alpha-proton abstraction step, showing that this step is driven by quantum mechanical hydrogen tunneling rather than the classical transition-state mechanism. Furthermore, an Arrhenius-type preexponential factor ratio approaching a transition-state value was obtained in the reaction of a mutant enzyme lacking the critical Asp. These results provide strong evidence for enzyme-enhanced hydrogen tunneling. X-ray crystallographic structures of the reaction intermediates revealed a small difference in the binding mode of distal parts of substrates, which would modulate hydrogen tunneling proceeding through either active or passive dynamics. | |||
Quantum mechanical hydrogen tunneling in bacterial copper amine oxidase reaction.,Murakawa T, Okajima T, Kuroda S, Nakamoto T, Taki M, Yamamoto Y, Hayashi H, Tanizawa K Biochem Biophys Res Commun. 2006 Apr 7;342(2):414-23. Epub 2006 Feb 8. PMID:16487484<ref>PMID:16487484</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
==See Also== | ==See Also== | ||
*[[Copper Amine Oxidase|Copper Amine Oxidase]] | *[[Copper Amine Oxidase|Copper Amine Oxidase]] | ||
== References == | |||
== | <references/> | ||
< | __TOC__ | ||
</StructureSection> | |||
[[Category: Arthrobacter globiformis]] | [[Category: Arthrobacter globiformis]] | ||
[[Category: Oxidoreductase]] | [[Category: Oxidoreductase]] | ||
Revision as of 07:43, 29 September 2014
Substrate Schiff-Base intermediate with tyramine in copper amine oxidase from Arthrobacter globiformisSubstrate Schiff-Base intermediate with tyramine in copper amine oxidase from Arthrobacter globiformis
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedA key step decisively affecting the catalytic efficiency of copper amine oxidase is stereospecific abstraction of substrate alpha-proton by a conserved Asp residue. We analyzed this step by pre-steady-state kinetics using a bacterial enzyme and stereospecifically deuterium-labeled substrates, 2-phenylethylamine and tyramine. A small and temperature-dependent kinetic isotope effect (KIE) was observed with 2-phenylethylamine, whereas a large and temperature-independent KIE was observed with tyramine in the alpha-proton abstraction step, showing that this step is driven by quantum mechanical hydrogen tunneling rather than the classical transition-state mechanism. Furthermore, an Arrhenius-type preexponential factor ratio approaching a transition-state value was obtained in the reaction of a mutant enzyme lacking the critical Asp. These results provide strong evidence for enzyme-enhanced hydrogen tunneling. X-ray crystallographic structures of the reaction intermediates revealed a small difference in the binding mode of distal parts of substrates, which would modulate hydrogen tunneling proceeding through either active or passive dynamics. Quantum mechanical hydrogen tunneling in bacterial copper amine oxidase reaction.,Murakawa T, Okajima T, Kuroda S, Nakamoto T, Taki M, Yamamoto Y, Hayashi H, Tanizawa K Biochem Biophys Res Commun. 2006 Apr 7;342(2):414-23. Epub 2006 Feb 8. PMID:16487484[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||||||