2qkh: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
Incretins, endogenous polypeptide hormones released in response to food | Incretins, endogenous polypeptide hormones released in response to food intake, potentiate insulin secretion from pancreatic beta cells after oral glucose ingestion (the incretin effect). This response is signaled by the two peptide hormones glucose-dependent insulinotropic polypeptide (GIP) (also known as gastric inhibitory polypeptide) and glucagon-like peptide 1 through binding and activation of their cognate class 2 G protein-coupled receptors (GPCRs). Because the incretin effect is lost or significantly reduced in patients with type 2 diabetes mellitus, glucagon-like peptide 1 and GIP have attracted considerable attention for their potential in antidiabetic therapy. A paucity of structural information precludes a detailed understanding of the processes of hormone binding and receptor activation, hampering efforts to develop novel pharmaceuticals. Here we report the crystal structure of the complex of human GIP receptor extracellular domain (ECD) with its agonist, the incretin GIP(1-42). The hormone binds in an alpha-helical conformation in a surface groove of the ECD largely through hydrophobic interactions. The N-terminal ligand residues would remain free to interact with other parts of the receptor. Thermodynamic data suggest that binding is concomitant with structural organization of the hormone, resulting in a complex mode of receptor-ligand recognition. The presentation of a well structured, alpha-helical ligand by the ECD is expected to be conserved among other hormone receptors of this class. | ||
==Disease== | |||
Known diseases associated with this structure: Pituitary ACTH-secreting adenoma OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=139360 139360]], Ventricular tachycardia, idiopathic OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=139360 139360]] | |||
==About this Structure== | ==About this Structure== | ||
| Line 13: | Line 16: | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Protein complex]] | [[Category: Protein complex]] | ||
[[Category: Demuth, H | [[Category: Demuth, H U.]] | ||
[[Category: Fanghanel, J.]] | [[Category: Fanghanel, J.]] | ||
[[Category: Kleinschmidt, M.]] | [[Category: Kleinschmidt, M.]] | ||
| Line 19: | Line 22: | ||
[[Category: Neumann, P.]] | [[Category: Neumann, P.]] | ||
[[Category: Parthier, C.]] | [[Category: Parthier, C.]] | ||
[[Category: Rahfeld, J | [[Category: Rahfeld, J U.]] | ||
[[Category: Rudolph, R.]] | [[Category: Rudolph, R.]] | ||
[[Category: Schlenzig, D.]] | [[Category: Schlenzig, D.]] | ||
[[Category: Stubbs, M | [[Category: Stubbs, M T.]] | ||
[[Category: QKH]] | [[Category: QKH]] | ||
[[Category: TAR]] | [[Category: TAR]] | ||
| Line 45: | Line 48: | ||
[[Category: transmembrane]] | [[Category: transmembrane]] | ||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 18:39:59 2008'' | ||
Revision as of 19:40, 21 February 2008
|
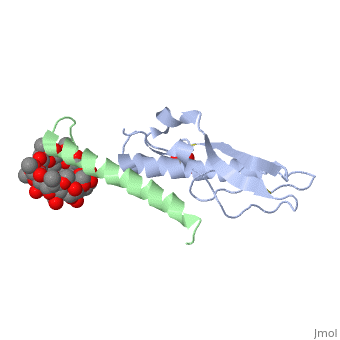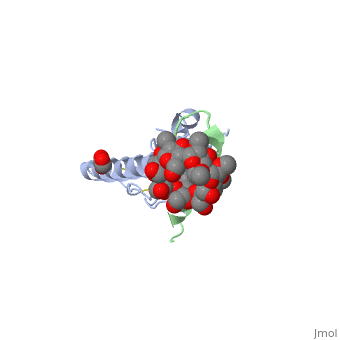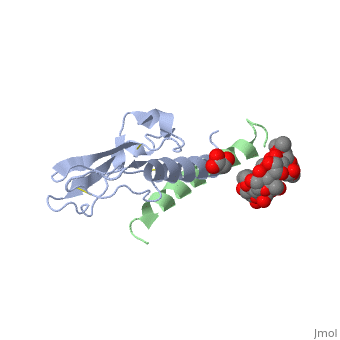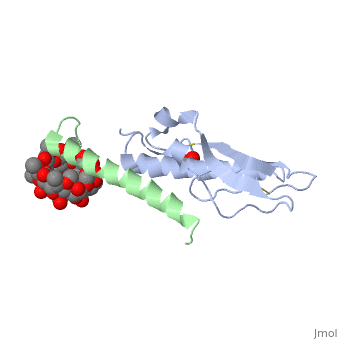
Crystal structure of the extracellular domain of human GIP receptor in complex with the hormone GIP
OverviewOverview
Incretins, endogenous polypeptide hormones released in response to food intake, potentiate insulin secretion from pancreatic beta cells after oral glucose ingestion (the incretin effect). This response is signaled by the two peptide hormones glucose-dependent insulinotropic polypeptide (GIP) (also known as gastric inhibitory polypeptide) and glucagon-like peptide 1 through binding and activation of their cognate class 2 G protein-coupled receptors (GPCRs). Because the incretin effect is lost or significantly reduced in patients with type 2 diabetes mellitus, glucagon-like peptide 1 and GIP have attracted considerable attention for their potential in antidiabetic therapy. A paucity of structural information precludes a detailed understanding of the processes of hormone binding and receptor activation, hampering efforts to develop novel pharmaceuticals. Here we report the crystal structure of the complex of human GIP receptor extracellular domain (ECD) with its agonist, the incretin GIP(1-42). The hormone binds in an alpha-helical conformation in a surface groove of the ECD largely through hydrophobic interactions. The N-terminal ligand residues would remain free to interact with other parts of the receptor. Thermodynamic data suggest that binding is concomitant with structural organization of the hormone, resulting in a complex mode of receptor-ligand recognition. The presentation of a well structured, alpha-helical ligand by the ECD is expected to be conserved among other hormone receptors of this class.
DiseaseDisease
Known diseases associated with this structure: Pituitary ACTH-secreting adenoma OMIM:[139360], Ventricular tachycardia, idiopathic OMIM:[139360]
About this StructureAbout this Structure
2QKH is a Protein complex structure of sequences from Homo sapiens with and as ligands. Full crystallographic information is available from OCA.
ReferenceReference
Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor., Parthier C, Kleinschmidt M, Neumann P, Rudolph R, Manhart S, Schlenzig D, Fanghanel J, Rahfeld JU, Demuth HU, Stubbs MT, Proc Natl Acad Sci U S A. 2007 Aug 28;104(35):13942-7. Epub 2007 Aug 21. PMID:17715056
Page seeded by OCA on Thu Feb 21 18:39:59 2008
Proteopedia Page Contributors and Editors (what is this?)Proteopedia Page Contributors and Editors (what is this?)
OCA- Pages with broken file links
- Homo sapiens
- Protein complex
- Demuth, H U.
- Fanghanel, J.
- Kleinschmidt, M.
- Manhart, S.
- Neumann, P.
- Parthier, C.
- Rahfeld, J U.
- Rudolph, R.
- Schlenzig, D.
- Stubbs, M T.
- QKH
- TAR
- Alternative splicing
- Cleavage on pair of basic residues
- Diabetes
- Ecd
- Extracellular domain
- G-protein coupled receptor
- Glugacon receptor family
- Glycoprotein
- Gpcr
- Helix formation
- Hormone recognition fold
- Hormone-gpcr complex
- Incretin
- Ligand binding domain
- Membrane
- Polymorphism
- Signaling protein/hormone complex
- Transducer
- Transmembrane