Lactose Permease: Difference between revisions
No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (46 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
[[Image:Lacpermmodel.jpg|thumb|left|Physical Model of Lactose Permease based on [[1pv7]] from the [http://cbm.msoe.edu/ MSOE Center for BioMolecular Modeling] ]] | |||
===Function of Lactose Permease=== | ===Function of Lactose Permease=== | ||
[[ | [[Lactose Permease]] or '''galactoside permease''' (PDB entry [[1pv7]]) is a transmembrane protein that facilitates the passage of lactose across the phospholipid bi-layer of the cell membrane. The transport mechanism used is an active co-transport that uses the inwardly directed H+ electrochemical gradient as its driving force. As a result, the lactose is accompanied from the periplasm to the cytoplasm of the cell by an H+ proton<ref>PMID:15950162</ref>. '''Fucose permease''' transports fucose across cell membrane. | ||
Lactose is a disaccharide carbohydrate found primarily in mammalian milk. The disaccharide consists of the monosacharides glucose and galactose. When the lactose is ingested and absorbed into the cell, the enzyme lactase breaks the disaccharide into its monosaccharide subunits. These are in turn used in the cellular respiration process and broken down further into energy for the cell. | Lactose is a disaccharide carbohydrate found primarily in mammalian milk. The disaccharide consists of the monosacharides glucose and galactose. When the lactose is ingested and absorbed into the cell, the enzyme lactase breaks the disaccharide into its monosaccharide subunits. These are in turn used in the cellular respiration process and broken down further into energy for the cell. | ||
Lactose permease belongs to the family of so called [[Major Facilitators]]. | |||
===Structure of Lactose Permease=== | ===Structure of Lactose Permease=== | ||
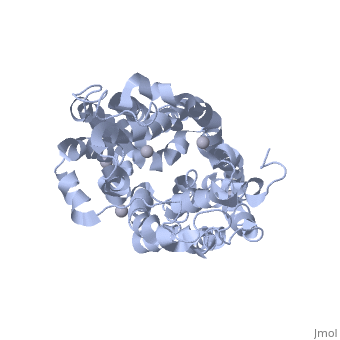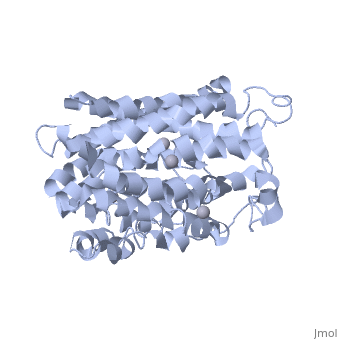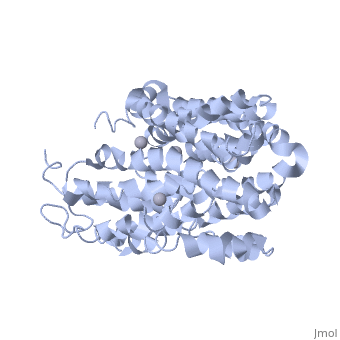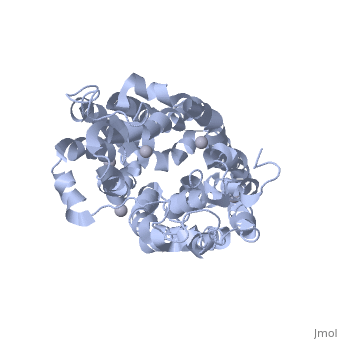
<applet load=" | <applet load="2cfp" size="400" color="white" frame="true" align="right" caption="E. coli lactose permease complex with Hg+2 ions (grey) [[2cfp]]" /> | ||
Lactose permease is a transmembrane protein consisting of N- and C- terminal domains, each with six transmembrane | <scene name='Lactose_Permease/Beginning/1'>Lactose permease</scene> is a transmembrane protein consisting of N- and C- terminal domains (depicted in this model by the blue and red hemispheres), each with six <scene name='Lactose_Permease/Backbone/3'>transmembrane helices</scene> symmetrically positioned within the permease. There are six sidechains that play an irreplaceable role in the active transport of lactose through the protein. Three of these sidechains, <scene name='Lactose_Permease/Glu126/3'>Glutamic Acid 126</scene>, <scene name='Lactose_Permease/Arg144/3'>Arginine 144</scene>, and <scene name='Lactose_Permease/Glu269/3'>Glutamic Acid 269</scene> have been shown to be crucial in substrate binding activities. <scene name='Lactose_Permease/Arg302/2'>Arginine 302</scene>, <scene name='Lactose_Permease/His322/2'>Histidine 322</scene>, and <scene name='Lactose_Permease/Glu325/2'>Glutamic Acid 325</scene> are known to play a significant role in proton translocation(moving the H+ proton) throughout the transport process. Additionally, there are two residues that are suspected to play an important role in the alignment of the galactopyranosyl end of the substrate. These are <scene name='Lactose_Permease/Cys148/2'>Cysteine 148</scene> and <scene name='Lactose_Permease/Trp151/2'>Tryptophan 151</scene>. | ||
These sidechains, which make up the active site of the protein, can be found within the large internal <scene name='Lactose_Permease/Cavity/2'>hydrophilic cavity</scene> of the lactose permease. It is here where the <scene name='Lactose_Permease/Sugar/2'>substrate</scene> is received for transport and it is the location from which it is deposited into the cell. The currently crystalized form of the permease is considered an 'inward-facing' conformation. This implies that the hydrophilic cavity mentioned previously is positioned with the opening towards the cytoplasm of the cell. Conversely, and outward-facing conformation would have the cavity facing the periplasm. | |||
{{Clear}} | |||
==3D structures of lactose permease== | |||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | |||
{{#tree:id=OrganizedByTopic|openlevels=0| | |||
*Lactose permease | |||
**[[2v8n]] - EcLACY – ''Escherichia coli''<br /> | |||
**[[2cfp]], [[2cfq]], [[1pv6]] - EcLACY (mutant) <br /> | |||
**[[2y5y]] – EcLACY (mutant) + affinity activator<br /> | |||
**[[4oaa]] - EcLACY (mutant) + galactose<br /> | |||
**[[1pv7]], [[4zyr]] - EcLACY (mutant) + galactoside<br /> | |||
**[[5gxb]], [[6c9w]] – EcLACY + nanobody<br /> | |||
**[[6vbg]] – EcLACY (mutant) + nanobody + galactoside derivative<br /> | |||
*Fucose permease | |||
**[[3o7q]] - EcFUCP<br /> | |||
**[[3o7p]] - EcFUCP (mutant) | |||
}} | |||
== References == | |||
<references/> | |||
==Additional Resources== | |||
For additional information, see: [[Membrane Channels & Pumps]] | |||
<br /> | |||
==External Links== | |||
===<font color = 'red'>MSOE Center for BioMolecular Modeling</font>=== | |||
[[Image:Center for BioMolecular Modeling Logo.jpg|left|200px]] | |||
The physical models shown on this page were designed and built by the MSOE Center for BioMolecular Modeling. For more information about physical protein modeling, visit the CBM web site at [http://cbm.msoe.edu/ http://cbm.msoe.edu/] . | |||
[[Category:Topic Page]] | |||
Latest revision as of 10:46, 15 June 2023

Function of Lactose PermeaseFunction of Lactose Permease
Lactose Permease or galactoside permease (PDB entry 1pv7) is a transmembrane protein that facilitates the passage of lactose across the phospholipid bi-layer of the cell membrane. The transport mechanism used is an active co-transport that uses the inwardly directed H+ electrochemical gradient as its driving force. As a result, the lactose is accompanied from the periplasm to the cytoplasm of the cell by an H+ proton[1]. Fucose permease transports fucose across cell membrane.
Lactose is a disaccharide carbohydrate found primarily in mammalian milk. The disaccharide consists of the monosacharides glucose and galactose. When the lactose is ingested and absorbed into the cell, the enzyme lactase breaks the disaccharide into its monosaccharide subunits. These are in turn used in the cellular respiration process and broken down further into energy for the cell.
Lactose permease belongs to the family of so called Major Facilitators.
Structure of Lactose PermeaseStructure of Lactose Permease
|
is a transmembrane protein consisting of N- and C- terminal domains (depicted in this model by the blue and red hemispheres), each with six symmetrically positioned within the permease. There are six sidechains that play an irreplaceable role in the active transport of lactose through the protein. Three of these sidechains, , , and have been shown to be crucial in substrate binding activities. , , and are known to play a significant role in proton translocation(moving the H+ proton) throughout the transport process. Additionally, there are two residues that are suspected to play an important role in the alignment of the galactopyranosyl end of the substrate. These are and .
These sidechains, which make up the active site of the protein, can be found within the large internal of the lactose permease. It is here where the is received for transport and it is the location from which it is deposited into the cell. The currently crystalized form of the permease is considered an 'inward-facing' conformation. This implies that the hydrophilic cavity mentioned previously is positioned with the opening towards the cytoplasm of the cell. Conversely, and outward-facing conformation would have the cavity facing the periplasm.
3D structures of lactose permease3D structures of lactose permease
Updated on 15-June-2023
ReferencesReferences
- ↑ Kaback HR. Structure and mechanism of the lactose permease. C R Biol. 2005 Jun;328(6):557-67. PMID:15950162 doi:10.1016/j.crvi.2005.03.008
Additional ResourcesAdditional Resources
For additional information, see: Membrane Channels & Pumps
External LinksExternal Links
MSOE Center for BioMolecular ModelingMSOE Center for BioMolecular Modeling

The physical models shown on this page were designed and built by the MSOE Center for BioMolecular Modeling. For more information about physical protein modeling, visit the CBM web site at http://cbm.msoe.edu/ .