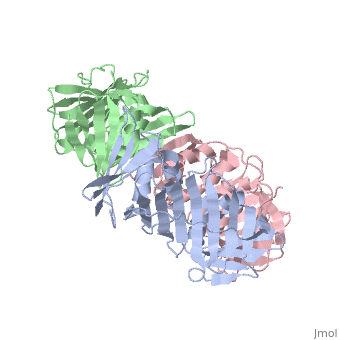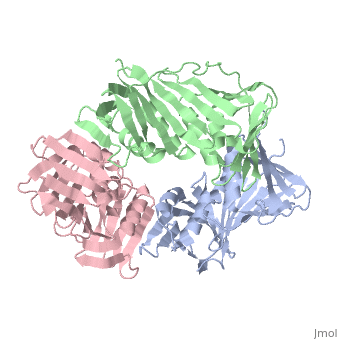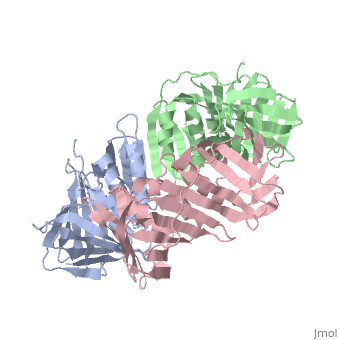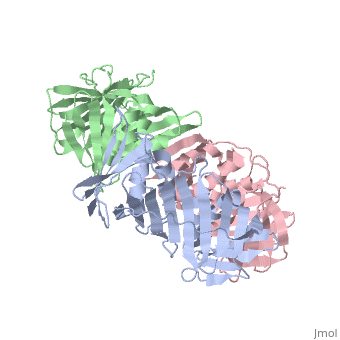2hik: Difference between revisions
No edit summary |
No edit summary |
||
| (10 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
< | ==heterotrimeric PCNA sliding clamp== | ||
<StructureSection load='2hik' size='340' side='right'caption='[[2hik]], [[Resolution|resolution]] 3.30Å' scene=''> | |||
You may | == Structural highlights == | ||
<table><tr><td colspan='2'>[[2hik]] is a 9 chain structure with sequence from [https://en.wikipedia.org/wiki/Saccharolobus_solfataricus Saccharolobus solfataricus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2HIK OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2HIK FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.3Å</td></tr> | |||
-- | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=MSE:SELENOMETHIONINE'>MSE</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2hik FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2hik OCA], [https://pdbe.org/2hik PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2hik RCSB], [https://www.ebi.ac.uk/pdbsum/2hik PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2hik ProSAT]</span></td></tr> | |||
</table> | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/hi/2hik_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview03.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2hik ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
DNA sliding clamps encircle DNA and provide binding sites for many DNA-processing enzymes. However, it is largely unknown how sliding clamps like proliferating cell nuclear antigen (PCNA) coordinate multistep DNA transactions. We have determined structures of Sulfolobus solfataricus DNA ligase and heterotrimeric PCNA separately by X-ray diffraction and in complex by small-angle X-ray scattering (SAXS). Three distinct PCNA subunits assemble into a protein ring resembling the homotrimeric PCNA of humans but with three unique protein-binding sites. In the absence of nicked DNA, the Sulfolobus solfataricus DNA ligase has an open, extended conformation. When complexed with heterotrimeric PCNA, the DNA ligase binds to the PCNA3 subunit and ligase retains an open, extended conformation. A closed, ring-shaped conformation of ligase catalyzes a DNA end-joining reaction that is strongly stimulated by PCNA. This open-to-closed switch in the conformation of DNA ligase is accommodated by a malleable interface with PCNA that serves as an efficient platform for DNA ligation. | |||
A flexible interface between DNA ligase and PCNA supports conformational switching and efficient ligation of DNA.,Pascal JM, Tsodikov OV, Hura GL, Song W, Cotner EA, Classen S, Tomkinson AE, Tainer JA, Ellenberger T Mol Cell. 2006 Oct 20;24(2):279-91. PMID:17052461<ref>PMID:17052461</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 2hik" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Proliferating cell nuclear antigen 3D structures|Proliferating cell nuclear antigen 3D structures]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
== | [[Category: Large Structures]] | ||
[[Category: Saccharolobus solfataricus]] | |||
[[Category: Ellenberger T]] | |||
== | [[Category: Pascal JM]] | ||
[[Category: Tsodikov OV]] | |||
[[Category: | |||
[[Category: | |||
[[Category: Ellenberger | |||
[[Category: Pascal | |||
[[Category: Tsodikov | |||
Latest revision as of 04:01, 21 November 2024
heterotrimeric PCNA sliding clampheterotrimeric PCNA sliding clamp
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedDNA sliding clamps encircle DNA and provide binding sites for many DNA-processing enzymes. However, it is largely unknown how sliding clamps like proliferating cell nuclear antigen (PCNA) coordinate multistep DNA transactions. We have determined structures of Sulfolobus solfataricus DNA ligase and heterotrimeric PCNA separately by X-ray diffraction and in complex by small-angle X-ray scattering (SAXS). Three distinct PCNA subunits assemble into a protein ring resembling the homotrimeric PCNA of humans but with three unique protein-binding sites. In the absence of nicked DNA, the Sulfolobus solfataricus DNA ligase has an open, extended conformation. When complexed with heterotrimeric PCNA, the DNA ligase binds to the PCNA3 subunit and ligase retains an open, extended conformation. A closed, ring-shaped conformation of ligase catalyzes a DNA end-joining reaction that is strongly stimulated by PCNA. This open-to-closed switch in the conformation of DNA ligase is accommodated by a malleable interface with PCNA that serves as an efficient platform for DNA ligation. A flexible interface between DNA ligase and PCNA supports conformational switching and efficient ligation of DNA.,Pascal JM, Tsodikov OV, Hura GL, Song W, Cotner EA, Classen S, Tomkinson AE, Tainer JA, Ellenberger T Mol Cell. 2006 Oct 20;24(2):279-91. PMID:17052461[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||