1ez0: Difference between revisions
New page: left|200px<br /><applet load="1ez0" size="450" color="white" frame="true" align="right" spinBox="true" caption="1ez0, resolution 2.10Å" /> '''CRYSTAL STRUCTURE OF... |
No edit summary |
||
| (19 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
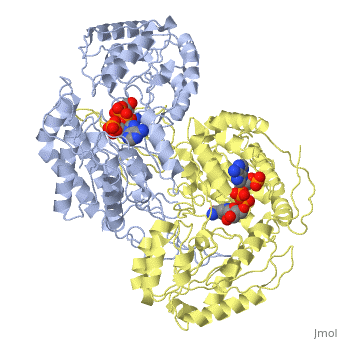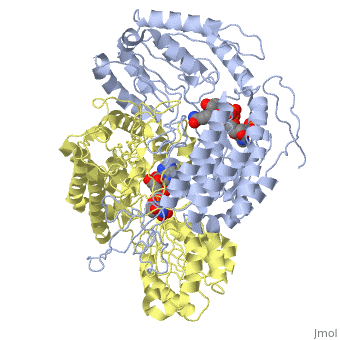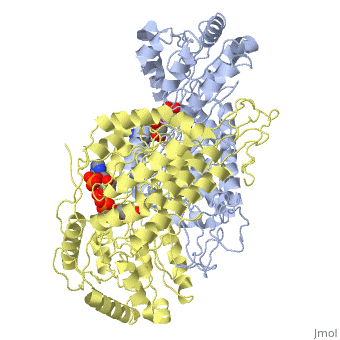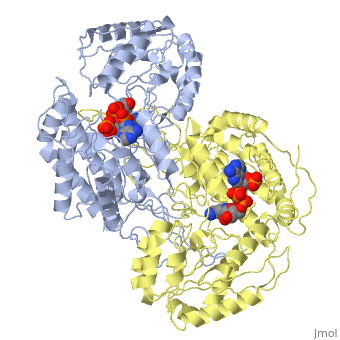
== | ==CRYSTAL STRUCTURE OF THE NADP+ DEPENDENT ALDEHYDE DEHYDROGENASE FROM VIBRIO HARVEYI.== | ||
Aldehyde dehydrogenase from the bioluminescent bacterium, Vibrio harveyi, catalyses the oxidation of long-chain aliphatic aldehydes to acids. The | <StructureSection load='1ez0' size='340' side='right'caption='[[1ez0]], [[Resolution|resolution]] 2.10Å' scene=''> | ||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1ez0]] is a 4 chain structure with sequence from [https://en.wikipedia.org/wiki/Vibrio_harveyi Vibrio harveyi]. This structure supersedes the now removed PDB entry [http://oca.weizmann.ac.il/oca-bin/send-pdb?obs=1&id=1co3 1co3]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1EZ0 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1EZ0 FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.1Å</td></tr> | |||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=NAP:NADP+NICOTINAMIDE-ADENINE-DINUCLEOTIDE+PHOSPHATE'>NAP</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1ez0 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1ez0 OCA], [https://pdbe.org/1ez0 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1ez0 RCSB], [https://www.ebi.ac.uk/pdbsum/1ez0 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1ez0 ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/ALDH_VIBHA ALDH_VIBHA] Catalyzes the oxidation of long-chain aliphatic aldehydes to acids. May be implicated in controlling luminescence as it catalyzes the oxidation of the fatty aldehyde substrate for the light-emitting reaction. | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/ez/1ez0_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1ez0 ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Aldehyde dehydrogenase from the bioluminescent bacterium, Vibrio harveyi, catalyses the oxidation of long-chain aliphatic aldehydes to acids. The enzyme is unique compared with other forms of aldehyde dehydrogenase in that it exhibits a very high specificity and affinity for the cofactor NADP(+). Structural studies of this enzyme and comparisons with other forms of aldehyde dehydrogenase provide the basis for understanding the molecular features that dictate these unique properties and will enhance our understanding of the mechanism of catalysis for this class of enzyme. The X-ray structure of aldehyde dehydrogenase from V. harveyi has been solved to 2.5-A resolution as a partial complex with the cofactor NADP(+) and to 2. 1-A resolution as a fully bound 'holo' complex. The cofactor preference exhibited by different forms of the enzyme is predominantly determined by the electrostatic environment surrounding the 2'-hydroxy or the 2'-phosphate groups of the adenosine ribose moiety of NAD(+) or NADP(+), respectively. In the NADP(+)-dependent structures the presence of a threonine and a lysine contribute to the cofactor specificity. In the V. harveyi enzyme an arginine residue (Arg-210) contributes to the high cofactor affinity through a pi stacking interaction with the adenine ring system of the cofactor. Further differences between the V. harveyi enzyme and other aldehyde dehydrogenases are seen in the active site, in particular a histidine residue which is structurally conserved with phosphorylating glyceraldehyde-3-phosphate dehydrogenase. This may suggest an alternative mechanism for activation of the reactive cysteine residue for nucleophilic attack. | |||
Crystal structure of the NADP+-dependent aldehyde dehydrogenase from Vibrio harveyi: structural implications for cofactor specificity and affinity.,Ahvazi B, Coulombe R, Delarge M, Vedadi M, Zhang L, Meighen E, Vrielink A Biochem J. 2000 Aug 1;349 Pt 3:853-61. PMID:10903148<ref>PMID:10903148</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
[[ | <div class="pdbe-citations 1ez0" style="background-color:#fffaf0;"></div> | ||
[[Category: | |||
==See Also== | |||
*[[Aldehyde dehydrogenase 3D structures|Aldehyde dehydrogenase 3D structures]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
[[Category: Large Structures]] | |||
[[Category: Vibrio harveyi]] | [[Category: Vibrio harveyi]] | ||
[[Category: Ahvazi | [[Category: Ahvazi B]] | ||
[[Category: Coulombe | [[Category: Coulombe R]] | ||
[[Category: Delarge | [[Category: Delarge M]] | ||
[[Category: Meighen | [[Category: Meighen E]] | ||
[[Category: Vedadi | [[Category: Vedadi M]] | ||
[[Category: Vrielink | [[Category: Vrielink A]] | ||
[[Category: Zhang | [[Category: Zhang L]] | ||
Latest revision as of 09:01, 9 August 2023
CRYSTAL STRUCTURE OF THE NADP+ DEPENDENT ALDEHYDE DEHYDROGENASE FROM VIBRIO HARVEYI.CRYSTAL STRUCTURE OF THE NADP+ DEPENDENT ALDEHYDE DEHYDROGENASE FROM VIBRIO HARVEYI.
Structural highlights
FunctionALDH_VIBHA Catalyzes the oxidation of long-chain aliphatic aldehydes to acids. May be implicated in controlling luminescence as it catalyzes the oxidation of the fatty aldehyde substrate for the light-emitting reaction. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedAldehyde dehydrogenase from the bioluminescent bacterium, Vibrio harveyi, catalyses the oxidation of long-chain aliphatic aldehydes to acids. The enzyme is unique compared with other forms of aldehyde dehydrogenase in that it exhibits a very high specificity and affinity for the cofactor NADP(+). Structural studies of this enzyme and comparisons with other forms of aldehyde dehydrogenase provide the basis for understanding the molecular features that dictate these unique properties and will enhance our understanding of the mechanism of catalysis for this class of enzyme. The X-ray structure of aldehyde dehydrogenase from V. harveyi has been solved to 2.5-A resolution as a partial complex with the cofactor NADP(+) and to 2. 1-A resolution as a fully bound 'holo' complex. The cofactor preference exhibited by different forms of the enzyme is predominantly determined by the electrostatic environment surrounding the 2'-hydroxy or the 2'-phosphate groups of the adenosine ribose moiety of NAD(+) or NADP(+), respectively. In the NADP(+)-dependent structures the presence of a threonine and a lysine contribute to the cofactor specificity. In the V. harveyi enzyme an arginine residue (Arg-210) contributes to the high cofactor affinity through a pi stacking interaction with the adenine ring system of the cofactor. Further differences between the V. harveyi enzyme and other aldehyde dehydrogenases are seen in the active site, in particular a histidine residue which is structurally conserved with phosphorylating glyceraldehyde-3-phosphate dehydrogenase. This may suggest an alternative mechanism for activation of the reactive cysteine residue for nucleophilic attack. Crystal structure of the NADP+-dependent aldehyde dehydrogenase from Vibrio harveyi: structural implications for cofactor specificity and affinity.,Ahvazi B, Coulombe R, Delarge M, Vedadi M, Zhang L, Meighen E, Vrielink A Biochem J. 2000 Aug 1;349 Pt 3:853-61. PMID:10903148[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences |
| ||||||||||||||||||