Ryanodine receptor: Difference between revisions
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
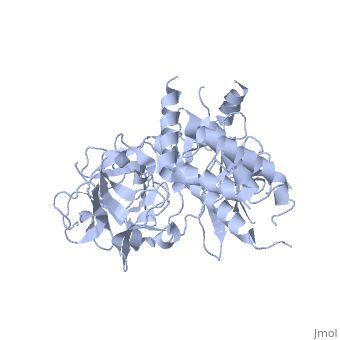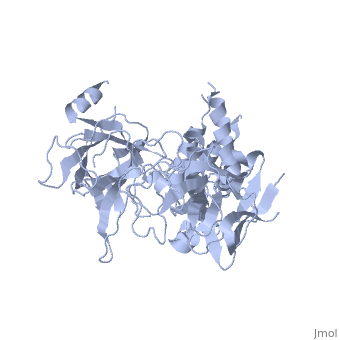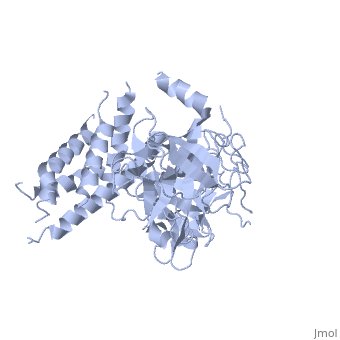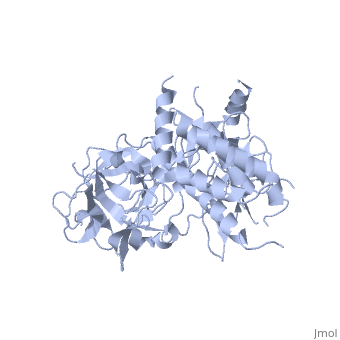
<StructureSection load='2xoa' size='340' side='right' caption='Rabbit ryanodine receptor 1 N-terminal (PDB code [[2xoa]])' scene=''> | <StructureSection load='2xoa' size='340' side='right' caption='Rabbit ryanodine receptor 1 N-terminal (PDB code [[2xoa]])' scene=''> | ||
__TOC__ | |||
== Function == | == Function == | ||
'''Ryanodine receptors''' (RyR) are the largest (over 5000 residues) ion channels and are responsible for the release of Ca+2 ions from the sarco/endoplasmic reticulum<ref>PMID:12270947</ref>. RyR is a homotetramer which opens upon binding of Ca+2. RyR is modulated by various ions, small molecules and proteins. The protein modulators include calmodulin, FKBP, protein phosphatase and protein kinase A. The C-terminal of RyR forms the transmembrane ion-conducting pore. The N-terminal of RyR named “foot”, includes a trefoil knot fold and interacts with the various modulators. The corners of the RYR cytoplasmic area are called “clamp” and undergo conformational change upon opening or closing of the channel. RYR domains include three SPRY domains. SPRY2 forms the link between the N terminal gating ring and the clamp region. RYR1 and RYR2 have several phosphorylation sites. Ryanodine is a plant alkaloid which binds to RyR with high affinity and locks the channel in an open state. There are 3 RyR isoforms.<br /> | '''Ryanodine receptors''' (RyR) are the largest (over 5000 residues) ion channels and are responsible for the release of Ca+2 ions from the sarco/endoplasmic reticulum<ref>PMID:12270947</ref>. RyR is a homotetramer which opens upon binding of Ca+2. RyR is modulated by various ions, small molecules and proteins. The protein modulators include calmodulin, FKBP, protein phosphatase and protein kinase A. The C-terminal of RyR forms the transmembrane ion-conducting pore. The N-terminal of RyR named “foot”, includes a trefoil knot fold and interacts with the various modulators. The corners of the RYR cytoplasmic area are called “clamp” and undergo conformational change upon opening or closing of the channel. RYR domains include three SPRY domains. SPRY2 forms the link between the N terminal gating ring and the clamp region. RYR1 and RYR2 have several phosphorylation sites. Ryanodine is a plant alkaloid which binds to RyR with high affinity and locks the channel in an open state. There are 3 RyR isoforms.<br /> | ||
| Line 14: | Line 15: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Latest revision as of 14:32, 31 December 2019
FunctionRyanodine receptors (RyR) are the largest (over 5000 residues) ion channels and are responsible for the release of Ca+2 ions from the sarco/endoplasmic reticulum[1]. RyR is a homotetramer which opens upon binding of Ca+2. RyR is modulated by various ions, small molecules and proteins. The protein modulators include calmodulin, FKBP, protein phosphatase and protein kinase A. The C-terminal of RyR forms the transmembrane ion-conducting pore. The N-terminal of RyR named “foot”, includes a trefoil knot fold and interacts with the various modulators. The corners of the RYR cytoplasmic area are called “clamp” and undergo conformational change upon opening or closing of the channel. RYR domains include three SPRY domains. SPRY2 forms the link between the N terminal gating ring and the clamp region. RYR1 and RYR2 have several phosphorylation sites. Ryanodine is a plant alkaloid which binds to RyR with high affinity and locks the channel in an open state. There are 3 RyR isoforms.
DiseaseMutations in RyR are associated with skeletal muscle disorders and triggered cardiac arrhythmia[2][3]. The N-terminal of RYR was found to be the disease hot spot of the molecule[4]. 3D Structures of ryanodine receptorRyanodine receptor 3D structures
|
| ||||||||||
ReferencesReferences
- ↑ Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002 Oct;82(4):893-922. PMID:12270947 doi:http://dx.doi.org/10.1152/physrev.00013.2002
- ↑ Sato K, Roesl C, Pollock N, Stowell KM. Skeletal muscle ryanodine receptor mutations associated with malignant hyperthermia showed enhanced intensity and sensitivity to triggering drugs when expressed in human embryonic kidney cells. Anesthesiology. 2013 Jul;119(1):111-8. doi: 10.1097/ALN.0b013e31828cebfe. PMID:23459219 doi:http://dx.doi.org/10.1097/ALN.0b013e31828cebfe
- ↑ McCarthy TV, Quane KA, Lynch PJ. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum Mutat. 2000;15(5):410-7. PMID:10790202 doi:<410::AID-HUMU2>3.0.CO;2-D http://dx.doi.org/10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D
- ↑ Tung CC, Lobo PA, Kimlicka L, Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 2010 Nov 3. PMID:21048710 doi:10.1038/nature09471