Tryptophan RNA-binding attenuation protein: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| Line 3: | Line 3: | ||
== Function == | == Function == | ||
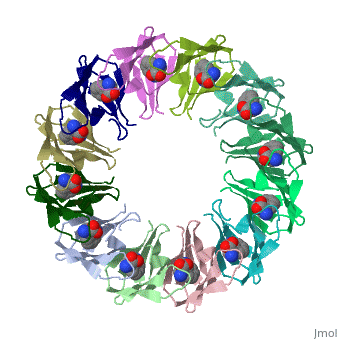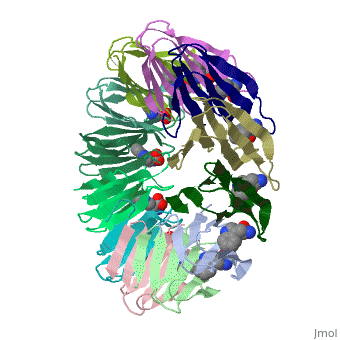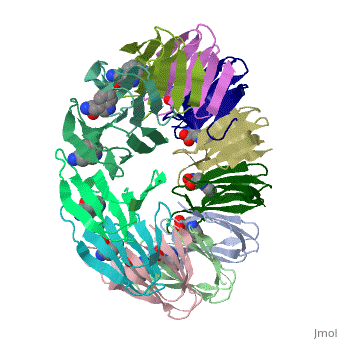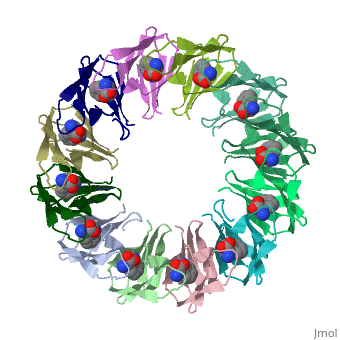
'''Tryptophan RNA-binding attenuation protein''' (TRAP) is a tryptophan-activated RNA-binding protein which regulates the expression of the tryptophan biosynthesis genes. Down regulation of Trp biosynthesis is observed upon binding of 11 tryptophan molecules to TRAP<ref>PMID:14687568</ref>. | '''Tryptophan RNA-binding attenuation protein''' (TRAP) or '''transcription attenuation protein MtrB''' is a tryptophan-activated RNA-binding protein which regulates the expression of the tryptophan biosynthesis genes. Down regulation of Trp biosynthesis is observed upon binding of 11 tryptophan molecules to TRAP<ref>PMID:14687568</ref>. | ||
== Structural highlights == | == Structural highlights == | ||
Latest revision as of 13:31, 21 December 2020
FunctionTryptophan RNA-binding attenuation protein (TRAP) or transcription attenuation protein MtrB is a tryptophan-activated RNA-binding protein which regulates the expression of the tryptophan biosynthesis genes. Down regulation of Trp biosynthesis is observed upon binding of 11 tryptophan molecules to TRAP[1]. Structural highlightsTRAP is composed of 11 identical subunits arranged in a . The [2]. Water molecules are shown as red spheres. |
| ||||||||||
3D Structures of tryptophan RNA-binding attenuation protein3D Structures of tryptophan RNA-binding attenuation protein
Updated on 21-December-2020
ReferencesReferences
- ↑ Li PT, Gollnick P. Characterization of a trp RNA-binding attenuation protein (TRAP) mutant with tryptophan independent RNA binding activity. J Mol Biol. 2004 Jan 16;335(3):707-22. PMID:14687568 doi:http://dx.doi.org/10.1016/S0022283603013846
- ↑ Heddle JG, Yokoyama T, Yamashita I, Park SY, Tame JR. Rounding up: Engineering 12-membered rings from the cyclic 11-mer TRAP. Structure. 2006 May;14(5):925-33. PMID:16698553 doi:http://dx.doi.org/10.1016/j.str.2006.03.013