Introduction to molecular visualization: Difference between revisions
Eric Martz (talk | contribs) |
Eric Martz (talk | contribs) |
||
| (119 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
''Molecular visualization'' means looking at molecular models in order to explore and understand them. Molecular visualization does not necessarily involve ''molecular modeling'', which means creating molecular models, or changing the composition or configurations of existing models. Here we will be dealing primarily with models of macromolecules (protein, DNA, RNA, or their complexes). | ''Molecular visualization'' means looking at molecular models in order to explore and understand them. Molecular visualization does not necessarily involve ''molecular modeling'', which means creating molecular models, or changing the composition or configurations of existing models. Here we will be dealing primarily with models of macromolecules (protein, DNA, RNA, or their complexes). | ||
__NOTOC__ | |||
==Representations of Molecular Models== | |||
===Atomic Representations=== | |||
<table align="right" class="wikitable" style="text-align:center;margin: 0px 0px 0px 16px;"><tr><td> | |||
[[Image:Cys-bs.png|200px]] | |||
</td><td> | |||
[[Image:Cys-sticks.png|200px]] | |||
</td><td> | |||
[[Image:Cys-spacefilled.png|200px]] | |||
</td></tr><tr><td> | |||
[http://biomodel.uah.es/en/model3/aa.htm Ball & Stick] | |||
</td><td> | |||
[http://biomodel.uah.es/en/model3/aa.htm Stick (Wireframe)] | |||
</td><td> | |||
[http://biomodel.uah.es/en/model3/aa.htm Spacefilling] | |||
</td></tr><tr><td colspan="3"> | |||
<span style="background-color:black;padding:5px 8px 1px 6px;font-size:140%;"><b><font color="#bbb">C</font> <font color="white">H</font> <font color="red">O</font> <font color="#58f">N</font> <font color="yellow">S</font></b></span> | |||
</td></tr></table> | |||
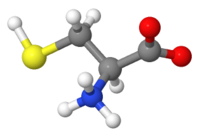
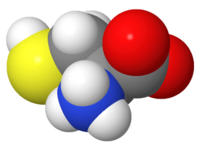
Atomic representations (displays, renderings) include '''ball-and-stick, stick (wireframe), and spacefilling'''. The 20 amino acids are [http://biomodel.uah.es/en/model3/aa.htm here represented in each of these 3 ways], and also illustrated in this [[Glycine|page about Glycine]]. These representations show positions of atoms and covalent bonds. [[Hydrogen in macromolecular models|Hydrogen]], shown | |||
in the images at right, is [[Hydrogen in macromolecular models|often missing]] in crystallographic models. Such representations are useful for looking at atomic detail, but become too cluttered to be useful for visualizing [[peptides]] or [[chains|protein chains]]. | |||
''Ball and stick'' is one option in the ''representations'' tab of Proteopedia's [[Scene Authoring Tools]]. Another is ''stick'', also called ''wireframe''. | |||
In [[FirstGlance in Jmol]], you can isolate a small portion of a large structure, and then display it as sticks (''Vines/Sticks'' in the ''Views'' tab). Or, rather than isolating it, which hides everything else, you can center it and then turn on slabbing. | |||
== | {{Clear}} | ||
===Slabbing=== | |||
<table align="right" class="wikitable" style="text-align:center;margin: 0px 0px 0px 16px;"><tr><td> | |||
[[Image:Slab-heme-sticks.png|200px]] | |||
</td><td> | |||
[[Image:Slab-core.png|230px]] | |||
</td></tr><tr><td> | |||
Slab showing heme ([http://FirstGlance.Jmol.Org/fg.htm?mol=1hho 1hho])<br><span style="background-color:black;padding:5px 8px 1px 6px;font-size:120%;"><b><font color="#bbb">C</font> <font color="red">O</font> <font color="#58f">N</font> <font color="orange">Fe</font></b></span> | |||
</td><td> | |||
Slab showing <font color="#909090">'''hydrophobic'''</font><br>core vs. <font color="#c040d0">'''polar'''</font> ([http://FirstGlance.Jmol.Org/fg.htm?mol=1pgb 1pgb]) | |||
</td></tr></table> | |||
A useful way to see atomic details of a small part of a large macromolecular model is to center the moiety of interest, and then cut away the front and back portions of the molecule. This is called ''slabbing'' since one is, in effect, looking at a slab cut out of the larger model. Slabbing is also useful to see buried structures and their environments, such as the hydrophobic core of a protein domain. Slabbing can be done using [[FirstGlance in Jmol]]: In the ''Views'' tab, use ''Center Atom'' to center the region of interest, and then click the ''Slab'' button. Further instructions will appear automatically. | |||
{{Clear}} | |||
===Simplified Schematic Representations=== | ===Simplified Schematic Representations=== | ||
====Backbones==== | |||
<table align="right" class="wikitable" style="text-align:center;margin: 0px 0px 0px 16px;"><tr><td> | |||
[[Image:Helix-bb-wf.png|200px]] | |||
</td><td> | |||
[[Image:Helix-backbone.png]] | |||
</td><td> | |||
[[Image:Helix-trace.png]] | |||
</td><td> | |||
[[Image:Helix-ribbon.png]] | |||
</td></tr><tr><td> | |||
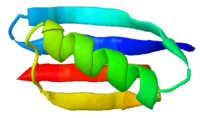
Alpha carbon<br>backbone trace ''simplifies''! | |||
</td><td> | |||
Alpha carbon<br>backbone trace | |||
</td><td> | |||
Smoothed<br>backbone trace | |||
</td><td> | |||
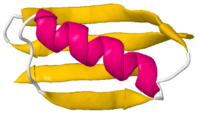
Ribbon<br>backbone trace | |||
</td></tr></table> | |||
Simplified representations of the polypeptide backbone or main chain, such as '''[[Backbone_representations|backbone traces or ribbons/cartoons]]''' are very helpful in understanding structure when it comes to large molecules such as proteins, DNA, RNA and their complexes. These representations are available under the ''representations'' tab in Proteopedia's [[Scene Authoring Tools]], as well as in the ''Views'' tab in [[FirstGlance in Jmol]]. | |||
{{Clear}} | |||
====Disulfide Bonds==== | |||
<table class="wikitable" style="text-align:center;margin: 0px 0px 0px 16px;"> | |||
<tr><td width="200"> | |||
[[Image:Ss1.png|200px]] | |||
</td><td width="180"> | |||
[[Image:Ss2.png|180px]] | |||
</td><td width="180"> | |||
[[Image:Ss3.png|180px]] | |||
</td><td width="160"> | |||
[[Image:Ss4.png|160px]] | |||
</td><td width="170"> | |||
[[Image:Ss5.png|170px]] | |||
</td></tr><tr><td> | |||
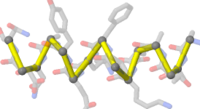
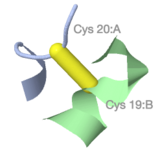
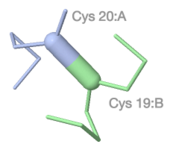
Atomic detail of a between-chain disulfide bond in [[9ins]]. | |||
<span style="background-color:black;padding:5px 8px 1px 6px;font-size:120%;"><b><font color="#bbb">C</font> <font color="red">O</font> <font color="#58f">N</font> <font color="yellow">S</font></b></span> | |||
</td><td> | |||
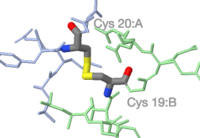
Protein chains | |||
<span style="background-color:black;padding:5px 8px 1px 6px;font-size:110%;"><b><font color="#acb8e2">A</font> <font color="#a0df99">B</font></b></span> | |||
simplified to backbone traces. | |||
</td><td> | |||
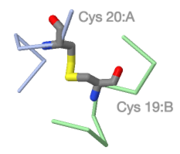
[[FirstGlance in Jmol|FirstGlance]] enlarges the sulfur-sulfur bond. | |||
</td><td> | |||
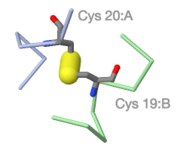
Schematic disulfide bridge connecting ribbon backbones. | |||
</td><td> | |||
Disulfide bridge colored by chain, an option in [[FirstGlance in Jmol|FirstGlance]]. | |||
</td></tr></table> | |||
[[FirstGlance in Jmol]] highlights disulfide bonds in one click (in its ''Tools'' tab), and has | |||
several options for rendering and coloring them. | |||
{{Clear}} | |||
==Color Schemes for Macromolecules== | ==Color Schemes for Macromolecules== | ||
<table align="right" class="wikitable" style="text-align:center;margin: 0px 0px 0px 16px;"><tr><td> | |||
[[Image:Domain-n2c-rainbow.png|200px]] | |||
</td><td> | |||
[[Image:Domain-ss.png|200px]] | |||
</td><td> | |||
[[Image:Domain-charge.png|200px]] | |||
</td><td> | |||
[[Image:Domain-composition.png|200px]] | |||
</td></tr><tr><td> | |||
N->C Rainbow ([https://bioinformatics.org/firstglance/fgij/fg.htm?mol=1pgb 1pgb]) | |||
{{Template:ColorKey_N2CRainbow}} | |||
</td><td> | |||
Secondary Structure<br> | |||
{{Template:ColorKey_Helix}},<br> | |||
{{Template:ColorKey_Strand}}, | |||
{{Template:ColorKey_Loop}}. | |||
</td><td> | |||
Amino Acid Charge<br> | |||
{{Template:ColorKey_Charge_Anionic}}<br>{{Template:ColorKey_Charge_Cationic}} | |||
</td><td> | |||
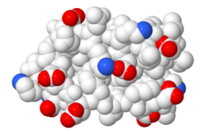
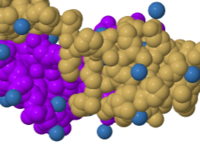
Composition ([https://bioinformatics.org/firstglance/fgij/fg.htm?mol=1d66 1d66])<br> | |||
{{Template:ColorKey Composition Protein}}, | |||
{{Template:ColorKey Composition DNA}}, | |||
{{Template:ColorKey Composition Solvent}} | |||
</td></tr></table> | |||
A set of standard color schemes for macromolecules, called [[DRuMS]], was released in 2000. These color schemes are offered on buttons in Proteopedia's [[Scene Authoring Tools]]. They derive in part from physical ball and stick models (called [[CPK|Corey-Pauling-Kolton or CPK]] models) that [[History of Macromolecular Visualization|pre-dated computer visualization]]. Those early colors for chemical elements (see examples above) were incorporated into early [[Molecular modeling and visualization software|Molecular Visualization Software]] such as [[Kinemages%2C_Mage_and_KiNG|Kinemages]], [[RasMol]], and [[Chime]]. The [[CPK]] colors for chemical elements, and the [[DRuMS]] color schemes were incorporated into the [http://jmol.sourceforge.net/jscolors/ color schemes] built into [[Jmol]], the visualization engine used in Proteopedia. | |||
See [[Help:Color Keys]] for color-key templates in Proteopedia. | |||
{{Clear}} | |||
==Visualizing Structural Features== | |||
===Overall Features=== | |||
<table align="right" class="wikitable" style="text-align:center;margin: 0px 0px 0px 16px;"><tr><td> | |||
[[Image:Firstglance-views-tab.png]] | |||
</td></tr><tr><td bgcolor="white"> | |||
''Views'' tab of [[FirstGlance in Jmol]] | |||
</td></tr></table> | |||
Combinations of representations and color schemes are useful to highlight | |||
# [[Protein primary, secondary, tertiary and quaternary structure|Secondary, tertiary, and quaternary structure]] | |||
# N- and C-terminal ends of protein chains; 5' and 3' ends of nucleic acid chains. | |||
# Distribution of polar (charged or uncharged: hydrophilic) vs. hydrophobic amino acids on the surface and in the core (using slabbing). | |||
# The distribution of positive and negative charges on the surface of a protein. | |||
# [[Introduction to Evolutionary Conservation|Evolutionary conservation]] to identify functional regions of proteins. | |||
# [[Jmol/Visualizing membrane position|Lipid bilayer boundaries]] for integral membrane proteins. | |||
Features 1-4 can be most easily displayed in the ''Views'' tab of [[FirstGlance in Jmol]]. Evolutionary conservation can sometimes be seen with [[How to see conserved regions|one click in Proteopedia]], or in other cases requires [[ConSurf Quick Analysis Procedure|submitting a job to the ConSurf server]]. Methods for visualizing lipid bilayer boundaries for integral membrane proteins are given in [[Jmol/Visualizing membrane position]]. | |||
For a specific protein, such features can be displayed by using Proteopedia's [[Scene Authoring Tools]] and then attached to green links when authoring a page. | |||
===Covalent and Non-Covalent Interactions=== | |||
<table align="right" class="wikitable" style="text-align:center;margin: 0px 0px 0px 16px;"><tr><td> | |||
[[Image:Firstglance-tools-tab.png|400px]] | |||
</td></tr><tr><td bgcolor="white"> | |||
''Tools'' tab of [[FirstGlance in Jmol]] | |||
</td></tr></table> | |||
[[FirstGlance in Jmol]] provides "one-click" routines, in its ''Tools'' tab, to highlight | |||
*Disulfide bonds | |||
*[[Salt bridges]] | |||
*[[Cation-pi interactions]] | |||
*Non-covalent interactions with any sub-structure that you select, using ''Contacts & Non-Covalent Interactions'' in the ''Tools'' tab. | |||
*Distances between any two atoms, and angles or dihedral angles defined by 3 or 4 atoms, using ''Distances/Angles'' in the ''Tools'' tab. | |||
*[[Crystal contacts]]. | |||
{{Clear}} | |||
==Obtaining Molecular Models== | |||
You can browse for molecular models at the [http://atlas.molviz.org Atlas of Macromolecules], the [http://pdb101.rcsb.org/ Molecule of the Month], or [https://web.expasy.org/spotlight/ Protein Spotlight]. | |||
[[How To Find A Structure]] explains an easy way to find a structure for a protein of interest, and how to choose the best one available when there is more than one. [[Empirical models]] are those determined by experimentation, notably [[X-ray diffraction]], [[solution nuclear magnetic resonance]], or [[electron cryomicroscopy]]. Empirical models are the most reliable, but one must pay attention to the [[Quality assessment for molecular models|quality of an empirical model]] since some are more reliable than others. | |||
Empirical models are available for only a small fraction of all proteins, probably <10%. When an empirical model is not available, [[AlphaFold]] has a proven track record of predicting protein structures correctly from their sequences, using artificial intelligence (AI). [[AlphaFold]] also reliably predicts the confidence of each part of a predicted structure. | |||
==See Also== | |||
*[[Help:Getting Started in Proteopedia]] | |||
*[[Help:Contents]] -- a list of many Help pages in Proteopedia. | |||
*[[History of Macromolecular Visualization]] | |||
*[[Molecular Sculpture]] | |||
[[Category: Alternate Renderings]] | |||
Latest revision as of 22:43, 4 December 2024
Molecular visualization means looking at molecular models in order to explore and understand them. Molecular visualization does not necessarily involve molecular modeling, which means creating molecular models, or changing the composition or configurations of existing models. Here we will be dealing primarily with models of macromolecules (protein, DNA, RNA, or their complexes).
Representations of Molecular ModelsRepresentations of Molecular Models
Atomic RepresentationsAtomic Representations
|
C H O N S | ||
Atomic representations (displays, renderings) include ball-and-stick, stick (wireframe), and spacefilling. The 20 amino acids are here represented in each of these 3 ways, and also illustrated in this page about Glycine. These representations show positions of atoms and covalent bonds. Hydrogen, shown in the images at right, is often missing in crystallographic models. Such representations are useful for looking at atomic detail, but become too cluttered to be useful for visualizing peptides or protein chains.
Ball and stick is one option in the representations tab of Proteopedia's Scene Authoring Tools. Another is stick, also called wireframe.
In FirstGlance in Jmol, you can isolate a small portion of a large structure, and then display it as sticks (Vines/Sticks in the Views tab). Or, rather than isolating it, which hides everything else, you can center it and then turn on slabbing.
SlabbingSlabbing
|
Slab showing heme (1hho) |
Slab showing hydrophobic |
A useful way to see atomic details of a small part of a large macromolecular model is to center the moiety of interest, and then cut away the front and back portions of the molecule. This is called slabbing since one is, in effect, looking at a slab cut out of the larger model. Slabbing is also useful to see buried structures and their environments, such as the hydrophobic core of a protein domain. Slabbing can be done using FirstGlance in Jmol: In the Views tab, use Center Atom to center the region of interest, and then click the Slab button. Further instructions will appear automatically.
Simplified Schematic RepresentationsSimplified Schematic Representations
BackbonesBackbones
|
Alpha carbon |
Alpha carbon |
Smoothed |
Ribbon |
Simplified representations of the polypeptide backbone or main chain, such as backbone traces or ribbons/cartoons are very helpful in understanding structure when it comes to large molecules such as proteins, DNA, RNA and their complexes. These representations are available under the representations tab in Proteopedia's Scene Authoring Tools, as well as in the Views tab in FirstGlance in Jmol.
Disulfide BondsDisulfide Bonds
|
Atomic detail of a between-chain disulfide bond in 9ins. C O N S |
Protein chains A B simplified to backbone traces. |
FirstGlance enlarges the sulfur-sulfur bond. |
Schematic disulfide bridge connecting ribbon backbones. |
Disulfide bridge colored by chain, an option in FirstGlance. |
FirstGlance in Jmol highlights disulfide bonds in one click (in its Tools tab), and has several options for rendering and coloring them.
Color Schemes for MacromoleculesColor Schemes for Macromolecules
|
N->C Rainbow (1pgb)
|
Secondary Structure |
Amino Acid Charge |
Composition (1d66) |
A set of standard color schemes for macromolecules, called DRuMS, was released in 2000. These color schemes are offered on buttons in Proteopedia's Scene Authoring Tools. They derive in part from physical ball and stick models (called Corey-Pauling-Kolton or CPK models) that pre-dated computer visualization. Those early colors for chemical elements (see examples above) were incorporated into early Molecular Visualization Software such as Kinemages, RasMol, and Chime. The CPK colors for chemical elements, and the DRuMS color schemes were incorporated into the color schemes built into Jmol, the visualization engine used in Proteopedia.
See Help:Color Keys for color-key templates in Proteopedia.
Visualizing Structural FeaturesVisualizing Structural Features
Overall FeaturesOverall Features
|
Views tab of FirstGlance in Jmol |
Combinations of representations and color schemes are useful to highlight
- Secondary, tertiary, and quaternary structure
- N- and C-terminal ends of protein chains; 5' and 3' ends of nucleic acid chains.
- Distribution of polar (charged or uncharged: hydrophilic) vs. hydrophobic amino acids on the surface and in the core (using slabbing).
- The distribution of positive and negative charges on the surface of a protein.
- Evolutionary conservation to identify functional regions of proteins.
- Lipid bilayer boundaries for integral membrane proteins.
Features 1-4 can be most easily displayed in the Views tab of FirstGlance in Jmol. Evolutionary conservation can sometimes be seen with one click in Proteopedia, or in other cases requires submitting a job to the ConSurf server. Methods for visualizing lipid bilayer boundaries for integral membrane proteins are given in Jmol/Visualizing membrane position.
For a specific protein, such features can be displayed by using Proteopedia's Scene Authoring Tools and then attached to green links when authoring a page.
Covalent and Non-Covalent InteractionsCovalent and Non-Covalent Interactions
|
Tools tab of FirstGlance in Jmol |
FirstGlance in Jmol provides "one-click" routines, in its Tools tab, to highlight
- Disulfide bonds
- Salt bridges
- Cation-pi interactions
- Non-covalent interactions with any sub-structure that you select, using Contacts & Non-Covalent Interactions in the Tools tab.
- Distances between any two atoms, and angles or dihedral angles defined by 3 or 4 atoms, using Distances/Angles in the Tools tab.
- Crystal contacts.
Obtaining Molecular ModelsObtaining Molecular Models
You can browse for molecular models at the Atlas of Macromolecules, the Molecule of the Month, or Protein Spotlight.
How To Find A Structure explains an easy way to find a structure for a protein of interest, and how to choose the best one available when there is more than one. Empirical models are those determined by experimentation, notably X-ray diffraction, solution nuclear magnetic resonance, or electron cryomicroscopy. Empirical models are the most reliable, but one must pay attention to the quality of an empirical model since some are more reliable than others.
Empirical models are available for only a small fraction of all proteins, probably <10%. When an empirical model is not available, AlphaFold has a proven track record of predicting protein structures correctly from their sequences, using artificial intelligence (AI). AlphaFold also reliably predicts the confidence of each part of a predicted structure.
See AlsoSee Also
- Help:Getting Started in Proteopedia
- Help:Contents -- a list of many Help pages in Proteopedia.
- History of Macromolecular Visualization
- Molecular Sculpture