Polygalacturonase: Difference between revisions
No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (28 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
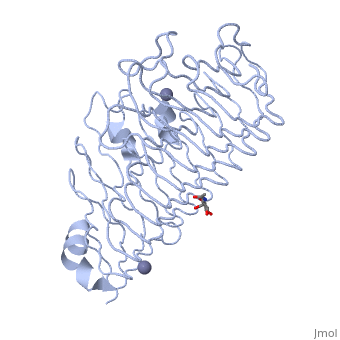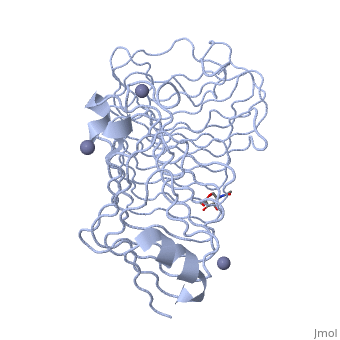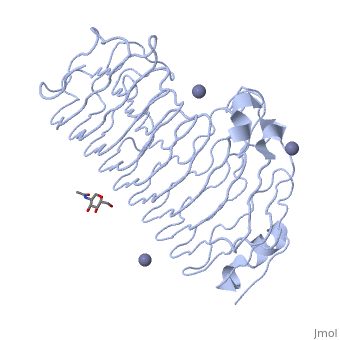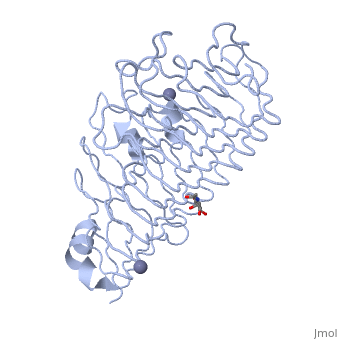
< | <StructureSection load='1czf' size='350' side='right' caption='Glycosylated structure of endo-polygalacturonase II complex with Zn+2 ion (grey) (PDB code [[1czf]])' scene=''> | ||
'''Polygalacturonases''' (PGs) catalyze the enzymatic depolymerization of pectates – polysaccharides that comprise the plant cell wall. Polymer disassembly of substrates by ''exo-'' and ''endo-'' PGs is carried out via a hydrolytic mechanism. Degradation of pectates in plant cell walls contributes to ripening of fruits, such as tomatoes and melons<ref>PMID:9625687</ref>. Microbial PGs have been identified to be a part of defense mechanisms because of their role in pathogen attack<ref name="crystal">PMID:9733763</ref>. | |||
PG's are found in bacteria, fungi, plants, and animals. Plant PG's are involved in fruit ripening. Bacteria and fungal PG are involved in plant pathogenesis, often acting as plant virulence factors and involved in some of the initial pathogenic effects through their action of degrading the plant cell wall. | |||
== Function == | == Function == | ||
Polygalacturonases hydrolyze α-(1-4) – glycosidic bonds between consecutive galacturonic acid residues in polygalacturonic acids. Structural variation has been identified among differing PGs depending on organismal origins and catalytic functions. For example, endo-polygalacturonases produced from <i>Erwinia carotovora </i> demonstrate functional similarity to pectate lyases in that they cleave polygalacturonic acids in a calcium-depended manner via β-elimination | Polygalacturonases hydrolyze α-(1-4) – glycosidic bonds between consecutive galacturonic acid residues in polygalacturonic acids. Structural variation has been identified among differing PGs depending on organismal origins and catalytic functions. For example, endo-polygalacturonases produced from <i>Erwinia carotovora </i> demonstrate functional similarity to pectate lyases in that they cleave polygalacturonic acids in a calcium-depended manner via β-elimination<ref name="crystal" />. | ||
One must distinguish between pectate and pectin. Pectate is a galacturonate polymer, pectin has a polygalacturonate backbone, but some of the monomers are methylesterified on the sixth carbon. PG acts on pectate, not pectin. | |||
== | == Structural highlights == | ||
The tertiary fold of PGs varies in its composition of coils, approximating at 10 coils in a right-handed parallel beta helix domain along with loop regions that together form the substrate-binding cleft, which appears to have a tunnel-like shape. The active site of PGs is found between the looped regions of the protein. Located within the looped regions are two conserved aspartate residues that are predicted to participate in catalytic activity<ref name="crystal" />. | |||
A right-handed parallel beta helix is a tertiary fold. The secondary structure is the beta and the alpha structure. If you describe how the secondary structure folds in space, that becomes tertiary structure. The secondary structural elements of the core fold of the proteins are only beta structure, the beta strands form parallel beta sheets. There are three main parallel beta sheets, PG's often have a smaller parallel beta sheet of only three-four beta strands. Nomenclature on how the sheets and turns are labeled are described in Yoder et al<ref name="yoder">PMID:8081738</ref>. | |||
Not all PGs have ten coils in the parallel beta helix. They usually have approximately 10 coils. [[Image:PBH2.jpg|200px|left|thumb| Nomenclature for structural elements of the parallel beta helix<ref name="yoder" />. PB1 is Parallel Beta Sheet 1, T1 is Turn 1, between PB1 and PB2., PB3]] With the parallel beta helix fold, the three major beta sheets are call PB1, PB2, and PB3. The turns between strands are Turn 1 (T1) between PB1 and PB2, T2 is the turn between PB2 and PB3, and T3 is the turn between PB3 and PB1 of the next coil. For your reference, this is illustrated in the figure below. | |||
</StructureSection> | |||
== | == 3D Structures of polygalacturonase == | ||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | |||
{{#tree:id=OrganizedByTopic|openlevels=0| | |||
*Polygalacturonase | |||
**[[2ci3]], [[1ib4]], [[1ia5]] – PGU – ''Aspergillus aculeatus'' <br /> | |||
**[[1bhe]] – PGU – ''Pectobacterium carotovorum'' <br /> | |||
*Endopolygalacturonase | |||
**[[7b7a]] – AtEPGU – ''Arabidopsis thaliana''<br /> | |||
**[[7b8b]] – AtEPGU2<br /> | |||
**[[2iq7]] – EPGU – ''Colletotrichum lupini'' <br /> | |||
**[[1hg8]] – EPGU – ''Fusarium moniliforme'' <br /> | |||
**[[1nhc]] – AnEPGU I – ''Aspergillus niger'' <br /> | |||
**[[1czf]] – AnEPGU II <br /> | |||
**[[6kve]] – TlEPGU – ''Talaromyces leycettanus''<br /> | |||
**[[6kvh]], [[7e56]] – TlEPGU (mutant)<br /> | |||
*Exopolygalacturonase | |||
**[[2uve]] – YeEXPGU – ''Yersinia enterocolitica'' <br /> | |||
**[[2uvf]] – YeEXPGU + digalacturonic acid <br /> | |||
}} | |||
== References == | == References == | ||
<references/> | <references/> | ||
Latest revision as of 12:49, 4 September 2023
Polygalacturonases (PGs) catalyze the enzymatic depolymerization of pectates – polysaccharides that comprise the plant cell wall. Polymer disassembly of substrates by exo- and endo- PGs is carried out via a hydrolytic mechanism. Degradation of pectates in plant cell walls contributes to ripening of fruits, such as tomatoes and melons[1]. Microbial PGs have been identified to be a part of defense mechanisms because of their role in pathogen attack[2]. PG's are found in bacteria, fungi, plants, and animals. Plant PG's are involved in fruit ripening. Bacteria and fungal PG are involved in plant pathogenesis, often acting as plant virulence factors and involved in some of the initial pathogenic effects through their action of degrading the plant cell wall. FunctionPolygalacturonases hydrolyze α-(1-4) – glycosidic bonds between consecutive galacturonic acid residues in polygalacturonic acids. Structural variation has been identified among differing PGs depending on organismal origins and catalytic functions. For example, endo-polygalacturonases produced from Erwinia carotovora demonstrate functional similarity to pectate lyases in that they cleave polygalacturonic acids in a calcium-depended manner via β-elimination[2]. One must distinguish between pectate and pectin. Pectate is a galacturonate polymer, pectin has a polygalacturonate backbone, but some of the monomers are methylesterified on the sixth carbon. PG acts on pectate, not pectin. Structural highlightsThe tertiary fold of PGs varies in its composition of coils, approximating at 10 coils in a right-handed parallel beta helix domain along with loop regions that together form the substrate-binding cleft, which appears to have a tunnel-like shape. The active site of PGs is found between the looped regions of the protein. Located within the looped regions are two conserved aspartate residues that are predicted to participate in catalytic activity[2]. A right-handed parallel beta helix is a tertiary fold. The secondary structure is the beta and the alpha structure. If you describe how the secondary structure folds in space, that becomes tertiary structure. The secondary structural elements of the core fold of the proteins are only beta structure, the beta strands form parallel beta sheets. There are three main parallel beta sheets, PG's often have a smaller parallel beta sheet of only three-four beta strands. Nomenclature on how the sheets and turns are labeled are described in Yoder et al[3]. Not all PGs have ten coils in the parallel beta helix. They usually have approximately 10 coils.  With the parallel beta helix fold, the three major beta sheets are call PB1, PB2, and PB3. The turns between strands are Turn 1 (T1) between PB1 and PB2, T2 is the turn between PB2 and PB3, and T3 is the turn between PB3 and PB1 of the next coil. For your reference, this is illustrated in the figure below.
|
| ||||||||||
3D Structures of polygalacturonase3D Structures of polygalacturonase
Updated on 04-September-2023
ReferencesReferences
- ↑ Hadfield KA, Bennett AB. Polygalacturonases: many genes in search of a function. Plant Physiol. 1998 Jun;117(2):337-43. PMID:9625687
- ↑ 2.0 2.1 2.2 Pickersgill R, Smith D, Worboys K, Jenkins J. Crystal structure of polygalacturonase from Erwinia carotovora ssp. carotovora. J Biol Chem. 1998 Sep 18;273(38):24660-4. PMID:9733763
- ↑ 3.0 3.1 Yoder MD, Lietzke SE, Jurnak F. Unusual structural features in the parallel beta-helix in pectate lyases. Structure. 1993 Dec 15;1(4):241-51. PMID:8081738