ABC transporter: Difference between revisions
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (25 intermediate revisions by 3 users not shown) | |||
| Line 4: | Line 4: | ||
== Function == | == Function == | ||
''' | '''ATP Binding Cassette''' (ABC) '''[[Transporters]]''' are ATP-dependent membrane proteins critical for most aspects of cell physiology, including the uptake of nutrients (importers) and elimination of waste products and energy generation (exporters) which are predominantly expressed in excretory organs, such as the liver, intestine, blood-brain barrier, blood-testes barrier, placenta, and kidney<ref name="Kidney"/><ref name="FourDomainsABCT"/>. There are many ABC Transporters in organisms, for instance, there are 28 in yeast, 58 in ''Caenorhabditis'', 51 in ''Drosophila'',129 in ''Arabadopsis'',and the 69 ABC transporters in ''E. coli'' account for almost 5% of its genomic coding capacity<ref name="EColi"/>. ABC transporter protein translocates substrates across membranes. It contains a Solute Binding Domain (SBD). '''CFTR''' (Cystic Fibrosis Transmembrane Regulator) translocates chloride and thiocyanate. It contains a nucleotide binding domain (NBD). In humans the ABC transporters are classified into subfamilies, i.e. ABCB6 is ABC subfamily B member 6. The '''Lipoprotein-release ABC transporter complex LolCDE''' is responsible for the transport of lipoproteins from the inner cell membrane to the outer membrane<ref>PMID:20149407</ref>.<br /> | ||
*For details of serotonin transporter see [[Serotonin Transporter]]<br /> | ABC Transporters have two main functionalities acting either as exporters or importers. '''ABC ''Exporters''''' release bound drugs to the extracellular environment, while '''ABC ''Importers''''' accept substrate molecules from their relevant substrate-binding proteins<ref name="biochembook"/>. For instance the Vitamin B12 transporter BtuCD (PDB [[1l7v]]) is a binding protein-dependent ABC transporter system that uses the power of ATP hydrolysis to pump vitamin B12 into the cytoplasm of E. coli<ref name="BtuCD-Ecoli"/>. <br /> | ||
*For details of sugar transporter see [[Glut3]] and [[GLUT4]]<br /> | *For details of '''serotonin transporter''' see [[Serotonin Transporter]]<br /> | ||
For details of | *For details of '''sugar transporter''' see [[GLUT1]],[[Glut3]], [[Sanbox glut3]], [[Glucose transport protein]] and [[GLUT4]]<br /> | ||
*For details of '''urea transporter''' see [[Urea transporter]]<br /> | |||
*For details of '''Cystic fibrosis transmembrane conductance regulator (CFTR)''' see [[Cystic fibrosis transmembrane conductance regulator (CFTR)]] and [[CFTR (Hebrew)]]<br /> | |||
*For details on '''plant abc transporter''' see [[Rice abc transporter]]<br /> | |||
*For details on '''dipeptide abc transporter''' see [[Periplasmic dipeptide-binding protein]]<br /> | |||
*For details on '''oligopeptide abc transporter''' see [[Oligopeptide-binding protein]]<br /> | |||
*For details on '''ABCG2 multi drug transporter''' see [[ABCG2 multidrug transporter]]<br /> | |||
== Structural highlights == | == Structural highlights == | ||
To achieve export, ABC transporters require a minimum of four domains. Two transmembrane domains (TMDs) form the ligand binding sites and provide specificity, and two NBDs bind and hydrolyze ATP to drive the trans-location of the bound ligand. The NBDs, but not the TMDs, are homologous throughout the family and have several characteristic motifs including the Walker A (GxxGxGKST) and B (ILLDEAT) motifs common to many nucleotide binding proteins and others like the ABC signature, stacking aromatic D, H, and Q loops, which are unique to the family<ref name="FourDomainsABCT"/>. | To achieve export, ABC transporters require a minimum of four domains. Two transmembrane domains (TMDs) form the ligand binding sites and provide specificity, and two NBDs bind and hydrolyze ATP to drive the trans-location of the bound ligand. The NBDs, but not the TMDs, are homologous throughout the family and have several characteristic motifs including the Walker A (GxxGxGKST) and B (ILLDEAT) motifs common to many nucleotide binding proteins and others like the ABC signature, stacking aromatic D, H, and Q loops, which are unique to the family<ref name="FourDomainsABCT"/>. | ||
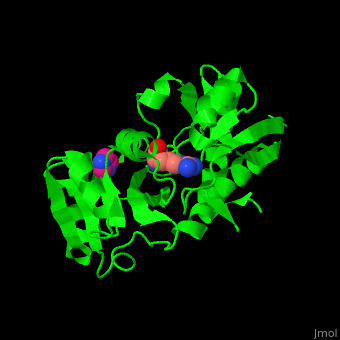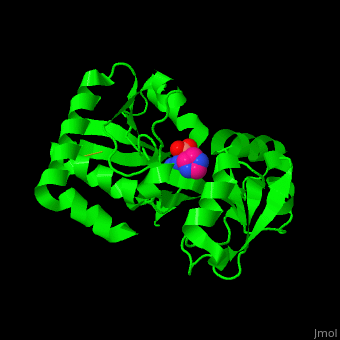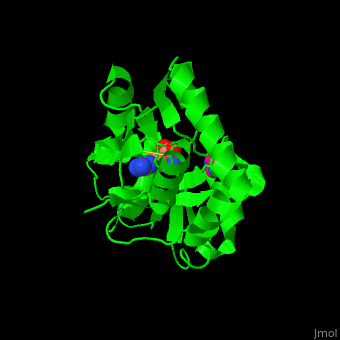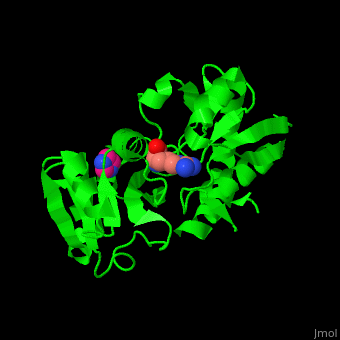
*<scene name='42/429005/Cv/ | *<scene name='42/429005/Cv/5'>Active site</scene> of Arginine ABC transporter complex with arginine (PDB code [[3tql]]). Water molecules are shown as red spheres. | ||
== Disease == | == Disease == | ||
There are currently 50 known ABC transporters in human. Of these there are 13 genetic diseases known to be associated with 14 of them. Among the diseases are cystic fibrosis, Stargardt disease, age-related macular degeneration and others. '''ABC Exporters''' use ATP to drive import and export functions providing multidrug resistance. In eukaryoles, for instance, ABC Transporters are problematic because they export therapeutic drugs such as those used in chemotherapy regimens, which must be changed frequently to avoid the rejection of the drugs<ref name="biochembook"/>. | There are currently 50 known ABC transporters in human. Of these there are 13 genetic diseases known to be associated with 14 of them. Among the diseases are cystic fibrosis, Stargardt disease, age-related macular degeneration and others. Mutations in CFTR lead to Cystic Fibrosis. '''ABC Exporters''' use ATP to drive import and export functions providing multidrug resistance. In eukaryoles, for instance, ABC Transporters are problematic because they export therapeutic drugs such as those used in chemotherapy regimens, which must be changed frequently to avoid the rejection of the drugs<ref name="biochembook"/>. | ||
== 3D Structures of ABC transporter == | == 3D Structures of ABC transporter == | ||
[[ABC transporter 3D structures]] | |||
</StructureSection> | |||
== References == | == References == | ||
{{reflist| | {{reflist| | ||
Latest revision as of 10:06, 20 May 2024
FunctionATP Binding Cassette (ABC) Transporters are ATP-dependent membrane proteins critical for most aspects of cell physiology, including the uptake of nutrients (importers) and elimination of waste products and energy generation (exporters) which are predominantly expressed in excretory organs, such as the liver, intestine, blood-brain barrier, blood-testes barrier, placenta, and kidney[1][2]. There are many ABC Transporters in organisms, for instance, there are 28 in yeast, 58 in Caenorhabditis, 51 in Drosophila,129 in Arabadopsis,and the 69 ABC transporters in E. coli account for almost 5% of its genomic coding capacity[3]. ABC transporter protein translocates substrates across membranes. It contains a Solute Binding Domain (SBD). CFTR (Cystic Fibrosis Transmembrane Regulator) translocates chloride and thiocyanate. It contains a nucleotide binding domain (NBD). In humans the ABC transporters are classified into subfamilies, i.e. ABCB6 is ABC subfamily B member 6. The Lipoprotein-release ABC transporter complex LolCDE is responsible for the transport of lipoproteins from the inner cell membrane to the outer membrane[4]. ABC Transporters have two main functionalities acting either as exporters or importers. ABC Exporters release bound drugs to the extracellular environment, while ABC Importers accept substrate molecules from their relevant substrate-binding proteins[5]. For instance the Vitamin B12 transporter BtuCD (PDB 1l7v) is a binding protein-dependent ABC transporter system that uses the power of ATP hydrolysis to pump vitamin B12 into the cytoplasm of E. coli[6].
Structural highlightsTo achieve export, ABC transporters require a minimum of four domains. Two transmembrane domains (TMDs) form the ligand binding sites and provide specificity, and two NBDs bind and hydrolyze ATP to drive the trans-location of the bound ligand. The NBDs, but not the TMDs, are homologous throughout the family and have several characteristic motifs including the Walker A (GxxGxGKST) and B (ILLDEAT) motifs common to many nucleotide binding proteins and others like the ABC signature, stacking aromatic D, H, and Q loops, which are unique to the family[2].
DiseaseThere are currently 50 known ABC transporters in human. Of these there are 13 genetic diseases known to be associated with 14 of them. Among the diseases are cystic fibrosis, Stargardt disease, age-related macular degeneration and others. Mutations in CFTR lead to Cystic Fibrosis. ABC Exporters use ATP to drive import and export functions providing multidrug resistance. In eukaryoles, for instance, ABC Transporters are problematic because they export therapeutic drugs such as those used in chemotherapy regimens, which must be changed frequently to avoid the rejection of the drugs[5]. 3D Structures of ABC transporter
|
| ||||||||||
ReferencesReferences
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedKidney - ↑ 2.0 2.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedFourDomainsABCT - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedEColi - ↑ Fenoll J, Ruiz E, Hellin P, Navarro S, Flores P. Solarization and biosolarization enhance fungicide dissipation in the soil. Chemosphere. 2010 Mar;79(2):216-20. doi: 10.1016/j.chemosphere.2010.01.034. Epub , 2010 Feb 10. PMID:20149407 doi:http://dx.doi.org/10.1016/j.chemosphere.2010.01.034
- ↑ 5.0 5.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedbiochembook - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedBtuCD-Ecoli