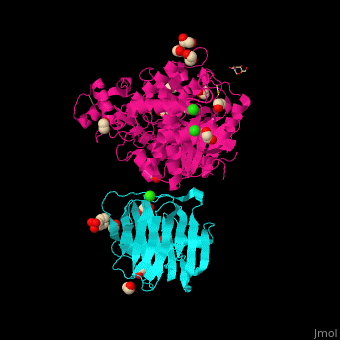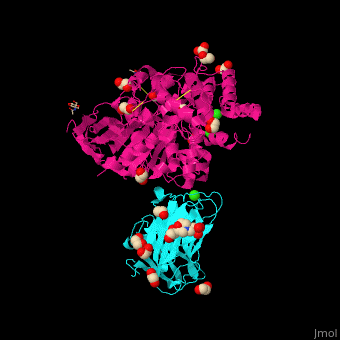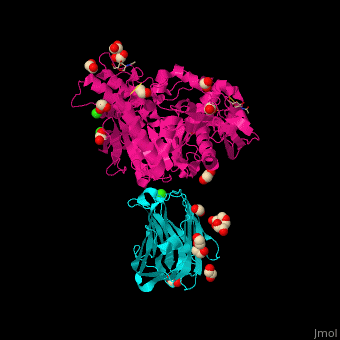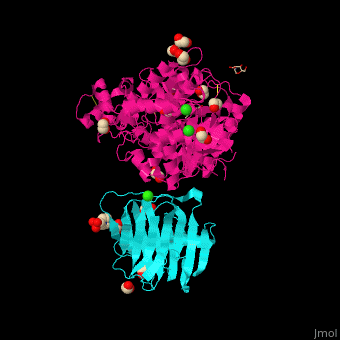Neurexin: Difference between revisions
Jump to navigation
Jump to search
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
<StructureSection load=' | <StructureSection load='' size='350' side='right' caption='Glycosylated rat neurexin 1α LNS domain (cyan) complex with neuroligin-4 cholinesterase domain (pink), MES, ethylene glycol, Cl- and Ca+2 ions (PDB entry [[2xb6]])' scene='42/424006/Cv/2'> | ||
__TOC__ | __TOC__ | ||
== Function == | == Function == | ||
| Line 11: | Line 11: | ||
== Structural highlights == | == Structural highlights == | ||
The surface interaction between NRXN and neuroligin is mediated by a Ca+2 ion<ref>PMID:20543817</ref>. | The <scene name='42/424006/Cv/7'>surface interaction between NRXN and neuroligin</scene> is mediated by a <scene name='42/424006/Cv/8'>Ca+2 ion</scene><ref>PMID:20543817</ref>. Water molecules are shown as red spheres. | ||
</StructureSection> | </StructureSection> | ||
| Line 22: | Line 22: | ||
*Neurexin | *Neurexin | ||
**[[5z8y]] – hNRXN-1b + LRRTM2 – human<br /> | |||
**[[3mw2]] – mNRXN-1α LNS domain+splice insert 4 – mouse<br /> | **[[3mw2]] – mNRXN-1α LNS domain+splice insert 4 – mouse<br /> | ||
**[[3bod]], [[3bop]] - mNRXN- | **[[3bod]], [[3bop]] - mβNRXN-1 residues 1132-1246 + mb-NRXN-2 residues 1277-1334<br /> | ||
**[[3mw4]] - mβNRXN-3 residues 854-1027<br /> | |||
**[[6pnp]], [[6pnq]] - mNRXN-1 LNS2 domain + neurexophilin-1<br /> | |||
**[[3mw3]], [[2r1b]] - rNRXN-1α LNS domain+splice insert 4 – rat<br /> | **[[3mw3]], [[2r1b]] - rNRXN-1α LNS domain+splice insert 4 – rat<br /> | ||
**[[2r16]] - rNRXN-1α LNS domain+Ca<br /> | **[[2r16]] - rNRXN-1α LNS domain+Ca<br /> | ||
**[[1c4r]] - rNRXN-1α LNS domain<br /> | **[[1c4r]] - rNRXN-1α LNS domain<br /> | ||
**[[ | **[[2r1d]] - rNRXN-1β LNS/LG domain<br /> | ||
**[[2h0b]] - bNRXN-1α LNS2 domain – bovine<br /> | **[[2h0b]] - bNRXN-1α LNS2 domain – bovine<br /> | ||
**[[6cw1]] - bNRXN-1 LNS2-LNS3 domains<br /> | |||
**[[3asi]] - bNRXN-1α LNS5-EGF3-LNS6 domains<br /> | **[[3asi]] - bNRXN-1α LNS5-EGF3-LNS6 domains<br /> | ||
**[[3poy]] - bNRXN-1α LNS2-LNS6 domains (mutant)<br /> | **[[3poy]] - bNRXN-1α LNS2-LNS6 domains (mutant)<br /> | ||
**[[3qcw]], [[3r05]] - bNRXN-1α LNS1-LNS6 domains (mutant)<br /> | **[[3qcw]], [[3r05]] - bNRXN-1α LNS1-LNS6 domains (mutant)<br /> | ||
*NRXN+Neuroligin | *NRXN+Neuroligin | ||
Latest revision as of 10:35, 7 November 2019
FunctionNeurexins (NRXN) are a family of presynaptic proteins which bind to the postsynaptic Neuroligin (NLGN) to form a complex which attaches neurons. The NRXN family contains 4 members which are numbered 1 to 4[1]. The NRXN-α contains 6 homologous LNS domains while the NRXN-β contains only LNS6. See: Neurodevelopmental Disorders Neuroligin-Neurexin Interaction RelevanceNRXN sequence variants may contribute to autism spectrum disorder and other cognitive diseases[2]. Structural highlightsThe is mediated by a [3]. Water molecules are shown as red spheres.
|
| ||||||||||
3D Structures of Neurexin3D Structures of Neurexin
07-November-2019
ReferencesReferences
- ↑ Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003 Jul;6(7):708-16. PMID:12796785 doi:http://dx.doi.org/10.1038/nn1074
- ↑ Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, Descartes M, Holt L, Braddock S, Troxell R, Kaplan L, Volkmar F, Klin A, Tsatsanis K, Harris DJ, Noens I, Pauls DL, Daly MJ, MacDonald ME, Morton CC, Quade BJ, Gusella JF. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008 Jan;82(1):199-207. doi: 10.1016/j.ajhg.2007.09.011. PMID:18179900 doi:http://dx.doi.org/10.1016/j.ajhg.2007.09.011
- ↑ Leone P, Comoletti D, Ferracci G, Conrod S, Garcia SU, Taylor P, Bourne Y, Marchot P. Structural insights into the exquisite selectivity of neurexin/neuroligin synaptic interactions. EMBO J. 2010 Jul 21;29(14):2461-71. Epub 2010 Jun 11. PMID:20543817 doi:10.1038/emboj.2010.123