Hepatocyte growth factor: Difference between revisions
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
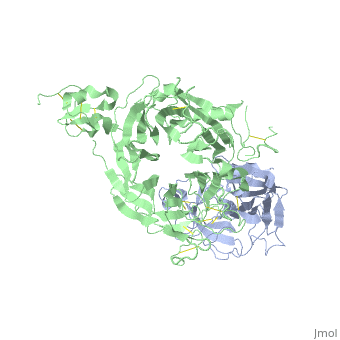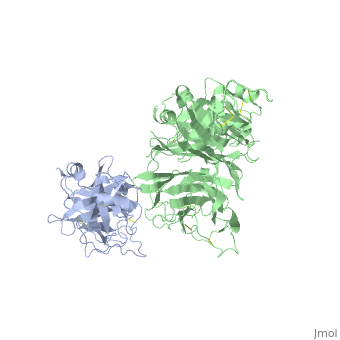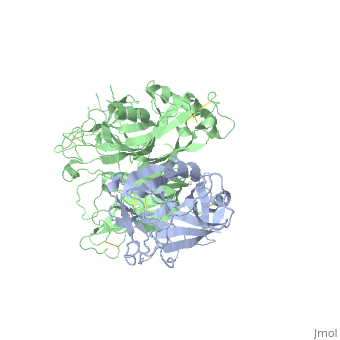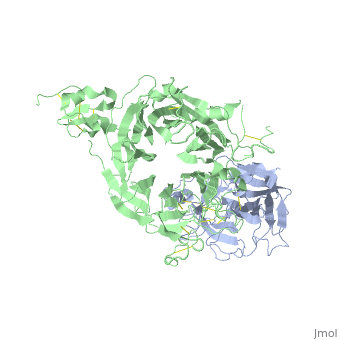
<StructureSection load='1shy' size='350' side='right' caption='Structure of human HGF β chain (grey) complex with HGF receptor Sema and ψ domains (green) (PDB entry [[1shy]])' scene=''> | <StructureSection load='1shy' size='350' side='right' caption='Structure of human HGF β chain (grey) complex with HGF receptor Sema and ψ domains (green) (PDB entry [[1shy]])' scene=''> | ||
== Function == | == Function == | ||
'''Hepatocyte growth factor''' (HGF) regulates cell growth, motility and morphogenesis. HGF binds to proto-oncogene c-Met receptor and activates a tyrosine kinase signaling cascade. HGF precursor is cleaved by serine protease to α (69 kD) and β (34 kD) chains which form a disulfide bond to produce the active heterodimer<ref>PMID:1838014</ref>. HGF α chain contains an N-terminal hairpin and 4 kringle domains. The kringle domain participates in protein-protein interaction and its structure is of a large loop which is stabilized by 3 Cys-Cys bonds. '''HGF NK1''' - a natural splice variant is comprised of residues 28-210 containing the N-terminus and the first kringle domain of HGF<ref>PMID:9488442</ref>. '''HGF NK2''' variant is comprised of residues 28-289 containing the N-terminus and the first 2 kringle domains of HGF. | '''Hepatocyte growth factor''' (HGF) regulates cell growth, motility and morphogenesis. HGF binds to proto-oncogene c-Met receptor and activates a tyrosine kinase signaling cascade. HGF precursor is cleaved by serine protease to α (69 kD) and β (34 kD) chains which form a disulfide bond to produce the active heterodimer<ref>PMID:1838014</ref>. '''HGF α chain''' contains an N-terminal hairpin and 4 kringle domains. The kringle domain participates in protein-protein interaction and its structure is of a large loop which is stabilized by 3 Cys-Cys bonds. '''HGF β chain''' is catalytically inactive serine protease-like. '''HGF NK1''' - a natural splice variant is comprised of residues 28-210 containing the N-terminus and the first kringle domain of HGF<ref>PMID:9488442</ref>. '''HGF NK2''' variant is comprised of residues 28-289 containing the N-terminus and the first 2 kringle domains of HGF. See also [[Hepatocyte growth factor receptor]]. | ||
== Relevance == | == Relevance == | ||
| Line 12: | Line 12: | ||
{{#tree:id=OrganizedByTopic|openlevels=0| | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
*HGF N terminal | *HGF full length 1-728 | ||
**[[7mo7]], [[7mo8]], [[7mo9]], [[7moa]], [[7mob]] – hHGF + hHGFR – human – Cryo EM <br /> | |||
*HGF N terminal 28-126 | |||
**[[2hgf]] – hHGF N terminal – human - NMR <br /> | **[[2hgf]] – hHGF N terminal – human - NMR <br /> | ||
| Line 18: | Line 22: | ||
**[[3hmr]] – mHGF N terminal – mouse <br /> | **[[3hmr]] – mHGF N terminal – mouse <br /> | ||
*HGF NK1 variant | *HGF NK1 variant 28-210 | ||
**[[1bht]], [[1nk1]], [[1gp9]], [[2qj2]], [[2qj4]] – hHGF NK1 <br /> | **[[1bht]], [[1nk1]], [[1gp9]], [[2qj2]], [[2qj4]] – hHGF NK1 <br /> | ||
**[[5cs1]], [[5cs5]], [[5cs9]], [[5coe]] – hHGF NK1 (mutant) <br /> | |||
**[[1gmn]], [[1gmo]], [[3mkp]] – hHGF NK1 (mutant) + heparin<br /> | **[[1gmn]], [[1gmo]], [[3mkp]] – hHGF NK1 (mutant) + heparin<br /> | ||
**[[5ct1]], [[5ct2]], [[5ct3]], [[5cs3]], [[5csq]], [[5cp9]] – hHGF NK1 (mutant) + inhibitor<br /> | |||
**[[4d3c]] – hHGF NK1 (mutant) + antibody<br /> | |||
*HGF NK2 variant | *HGF NK2 variant 28-289 | ||
**[[3hn4]] – hHGF NK2 <br /> | **[[3hn4]] – hHGF NK2 <br /> | ||
| Line 29: | Line 36: | ||
**[[4iua]] – mHGF NK2 (mutant) <br /> | **[[4iua]] – mHGF NK2 (mutant) <br /> | ||
*HGF α chain (69 kD) | * HGF α chain (69 kD) 495-728 | ||
**[[ | **[[1si5]] – hHGF α chain (mutant) <br /> | ||
**[[1shy]] – hHGF α chain (mutant) + HGF receptor Sema and ψ domains<br /> | |||
*HGF β chain | *HGF β chain 25-567 | ||
**[[ | **[[4o3t]], [[4o3u]] – hHGF β chain (mutant) + HGF receptor Sema and ψ domains + zymogen activator peptide<br /> | ||
*HGF α+β chain | *HGF α+β chain | ||
**[[4k3j]] – hHGF α (mutant) + β + antibody <br /> | **[[4k3j]] – hHGF α (mutant) + β + antibody <br /> | ||
**[[6lz9]] – hHGF α (mutant) + K4 domain 388-494 + antibody <br /> | |||
}} | |||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Latest revision as of 09:57, 16 May 2023
FunctionHepatocyte growth factor (HGF) regulates cell growth, motility and morphogenesis. HGF binds to proto-oncogene c-Met receptor and activates a tyrosine kinase signaling cascade. HGF precursor is cleaved by serine protease to α (69 kD) and β (34 kD) chains which form a disulfide bond to produce the active heterodimer[1]. HGF α chain contains an N-terminal hairpin and 4 kringle domains. The kringle domain participates in protein-protein interaction and its structure is of a large loop which is stabilized by 3 Cys-Cys bonds. HGF β chain is catalytically inactive serine protease-like. HGF NK1 - a natural splice variant is comprised of residues 28-210 containing the N-terminus and the first kringle domain of HGF[2]. HGF NK2 variant is comprised of residues 28-289 containing the N-terminus and the first 2 kringle domains of HGF. See also Hepatocyte growth factor receptor. RelevanceHigh levels of HGF are associated with liver diseases[3], lung diseases, acute cardiac infraction, vascular diseases, renal failure, neurologic diseases like Alzheimer Disease, pancreatic diseases, several types of cancer and diabetes type II[4][5]. HGF administration can reverse liver chirrosis[6]. |
| ||||||||||
3D structures of hepatocyte growth factor3D structures of hepatocyte growth factor
Updated on 16-May-2023
ReferencesReferences
- ↑ Nakamura T. Structure and function of hepatocyte growth factor. Prog Growth Factor Res. 1991;3(1):67-85. PMID:1838014
- ↑ Jakubczak JL, LaRochelle WJ, Merlino G. NK1, a natural splice variant of hepatocyte growth factor/scatter factor, is a partial agonist in vivo. Mol Cell Biol. 1998 Mar;18(3):1275-83. PMID:9488442
- ↑ Shiota G, Okano J, Kawasaki H, Kawamoto T, Nakamura T. Serum hepatocyte growth factor levels in liver diseases: clinical implications. Hepatology. 1995 Jan;21(1):106-12. PMID:7806142
- ↑ Anan F, Masaki T, Yonemochi H, Takahashi N, Nakagawa M, Eshima N, Saikawa T, Yoshimatsu H. Hepatocyte growth factor levels are associated with the results of 123I-metaiodobenzylguanidine myocardial scintigraphy in patients with type 2 diabetes mellitus. Metabolism. 2009 Feb;58(2):167-73. doi: 10.1016/j.metabol.2008.09.009. PMID:19154948 doi:http://dx.doi.org/10.1016/j.metabol.2008.09.009
- ↑ Shiota G, Okano J, Kawasaki H, Kawamoto T, Nakamura T. Serum hepatocyte growth factor levels in liver diseases: clinical implications. Hepatology. 1995 Jan;21(1):106-12. PMID:7806142
- ↑ Funakoshi H, Nakamura T. Hepatocyte growth factor: from diagnosis to clinical applications. Clin Chim Acta. 2003 Jan;327(1-2):1-23. PMID:12482615