Beta2 adrenergic receptor-Gs protein complex: Difference between revisions
Initial version |
No edit summary |
||
| (93 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
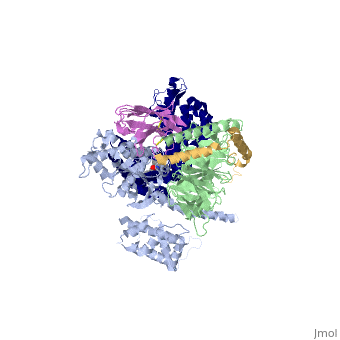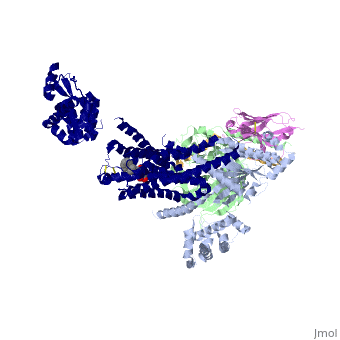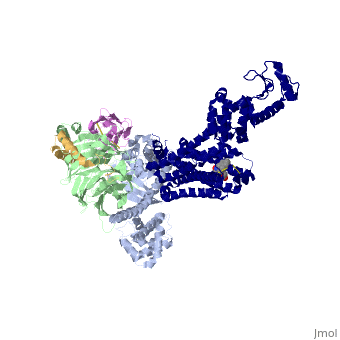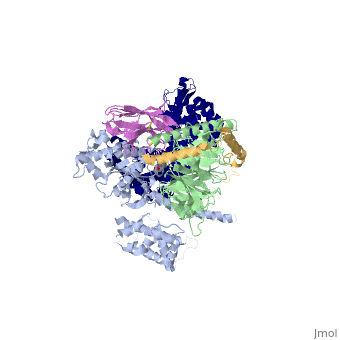
<StructureSection load='3sn6' size='340' side='right' caption='Adrenergic receptor (blue) complex with G protein α subunit (grey), β subunit (green), γ-2 subunit (gold), antibody fragment (Orchid) and benzoxazin derivative [[3sn6]], [[Resolution|resolution]] 3.20Å' scene=''> | |||
<StructureSection load='3sn6' size='340' side='right' caption='[[3sn6]], [[Resolution|resolution]] 3.20Å' scene=''> | |||
== | ==Introduction== | ||
[[G protein-coupled receptors]] (GPCRs) are a large family of protein receptors, which have seven-transmembrane helices and are found over a large array of eukaryotic cells. These receptors take a major part in a multitude of signal transduction pathways, including amongst others responses to hormones and neurotransmitters, sensing light, taste and smell, and many more. These receptors are also involved in many different types of diseases and are the target of almost 50% of current medical drugs. | |||
The [[Beta-2 Adrenergic Receptors]] are a type of GPCR that are activated by catecholamine hormone ligands such as adrenaline (epinephrine). These receptors are responsible for many of the adrenaline related (“fight-or-flight”) responses and functions, and are used as a common model system for the GPCR family. | |||
GPCRs bind their ligand and [[Group:SMART:A Physical Model of the β2-Adrenergic Receptor|overcome a conformational change]] that activates an attached [[Guanine nucleotide-binding protein]] (G protein) and allows it to detach from the cellular end of the receptor and start the different signal transduction pathways. | |||
G proteins<ref>https://en.wikipedia.org/wiki/G_protein</ref> are a family of proteins that act as molecular switches inside cells. G proteins belong to the larger group of enzymes called GTPases<ref>https://en.wikipedia.org/wiki/GTPase</ref>, and appear either as monomeric small GTPases<ref>https://en.wikipedia.org/wiki/Small_GTPase</ref>, or as heterotrimeric G protein complexes<ref>https://en.wikipedia.org/wiki/Heterotrimeric_G_protein</ref> that are made up of alpha (α), beta (β) and gamma (γ) subunits<ref>doi:10.1093/dnares/7.2.111</ref>. When they are bound to guanosine triphosphate ([https://en.wikipedia.org/wiki/Guanosine_triphosphate GTP]), they are 'on', and when they are bound to guanosine diphosphate ([https://en.wikipedia.org/wiki/Guanosine_diphosphate GDP]), they are 'off'. | |||
Since these receptors have seven transmembrane helices as well as inner and outer cell regions, they are very difficult to purify and crystallize. Some crystal structures have been determined for the inactive receptors as well as for the G proteins that they bind. PDB entry 3SN6 is the first structure of the full complex of the Beta 2 Adrenergic Receptor bound to Gs in their active state, and it provides the first high-resolution insight into the mechanism of signal transduction across the plasma membrane by a GPCR. | |||
== | == Complex structure == | ||
The overall structure shows the <scene name='70/701430/Receptor/1'>β2AR receptor</scene> (dark blue) bound to an agonist (in spheres) along with a <scene name='70/701430/Lysozyme/1'>T4</scene> [https://en.wikipedia.org/wiki/Lysozyme lysozyme] fused to its amino terminus in order to facilitate crystallization. The receptor interacts with <scene name='70/701430/Galpha/1'>Gαs</scene> (light blue). Gαs together with <scene name='70/701430/Gbeta/1'>Gβ</scene> (light green) and <scene name='70/701430/Ggamma/1'>Gγ</scene> (gold) constitute the heterotrimeric G protein Gs. A Gs-binding [https://en.wikipedia.org/wiki/Single-domain_antibody nanobody] <scene name='70/701430/Nanobody/1'>(in pink)</scene>, which also facilitates crystallization, binds the G protein between the α and β subunits. | |||
{{Template:Button Toggle Animation2}} | |||
== G-Protein-GPCR Interactions == | |||
The α5-helix of Gαs <scene name='70/701430/Docking/2'>docks</scene> into a cavity formed on the intracellular side of the receptor by the opening of the transmembrane helices. Within the transmembrane core, the interactions are primarily non-polar - an exception involves <scene name='70/701430/Receptor_g_protein_interaction/4'>packing</scene> of Tyr 391 of the α5-helix against Arg 131 of the conserved DRY sequence in TM3. Arg 131 also packs against Tyr 326 of the conserved NPxxY sequence in TM7. As the α5-helix exits the receptor it forms a network of polar interactions with TM5 and TM3. Receptor residues Thr 68 and Asp 130 interact with the ICL2 helix of the β2AR via Tyr 141, positioning the helix so that Phe 139 of the receptor <scene name='70/701430/Receptor_gprotein_interaction2/1'>docks into</scene> a hydrophobic pocket on the G protein surface, thereby structurally linking receptor–G protein interactions with the highly conserved DRY motif of the β2AR. | |||
{{Template:Button Toggle Animation2}} | |||
== G-Protein Activation Cycle == | |||
[[Image:Img.JPG|600px|G protein cycle for the β2AR–Gs complex. Reprinted by permission from Macmillan Publishers Ltd on behalf of Cancer Research UK: Nature 477, 549–555, copyright 2011]] | |||
The figure shows the G Protein cycle<ref>doi:10.1038/nature10361</ref> - an extracellular agonist binding to the β2AR leads to <scene name='70/701430/Receptor_morphing_animation/2'>conformational rearrangements</scene> of the cytoplasmic ends of transmembrane segments that enable the Gs heterotrimer to bind the receptor. GDP is released from the α subunit upon formation of β2AR–Gs complex. The GTP binds to the nucleotide-free α subunit resulting in dissociation of the α and βγ subunits from the receptor. The subunits regulate their respective effector proteins adenylyl cyclase (AC) and Ca2+ channels. The Gs heterotrimer reassembles from α and βγ subunits following hydrolysis of GTP to GDP in the α subunit. | |||
{{Template:Button Toggle Animation2}} | |||
== G-Protein variability == | |||
The Gαs subunit consists of two domains, the <scene name='70/701430/Alpharas/2'>Ras domain (GαsRas)</scene> and the <scene name='70/701430/Alphahelical/2'>α-helical domain (GαsAH)</scene>. A previous structure of a GTPγS bound (i.e. active, "turned on") Gαs protein (PDB ID: [http://www.rcsb.org/pdb/explore/explore.do?structureId=1AZT 1AZT]) showed that both domains are involved in nucleotide binding, as the nucleotide-binding pocket of the Gαs subunit is formed by the interface between GαsRas and GαsAH<ref>doi:10.1126/science.278.5345.1943</ref>. It was also previously known that the GsαAH domain has a variable position relative to the GsαRas domain between this GTP bound (active) state and the nucleotide free state<ref>DOI:10.1126/science.8266082</ref><ref>doi:10.1073/pnas.1105810108</ref><ref>doi:10.1073/pnas.1113645108</ref><ref>doi:10.1038/nature10488</ref>. However, the β2AR–Gs complex structure of the receptor attached to the empty (no guanosine phosphate attached) G protein enabled comparing it to the active (GTP bound) structure and by that showing <scene name='70/701430/Gamorph/2'>how large this displacement is</scene> - this is probably the most surprising observation arising from the β2AR–Gs complex. | |||
{{Template:Button Toggle Animation2}} | |||
==See Also== | ==See Also== | ||
| Line 35: | Line 46: | ||
*[[User:Wayne Decatur/UNH BCHEM833 Structural Analysis Workshop Session Fall 2012|User:Wayne Decatur/UNH BCHEM833 Structural Analysis Workshop Session Fall 2012]] | *[[User:Wayne Decatur/UNH BCHEM833 Structural Analysis Workshop Session Fall 2012|User:Wayne Decatur/UNH BCHEM833 Structural Analysis Workshop Session Fall 2012]] | ||
*[[User:Wayne Decatur/UNH BCHEM833 Structural Proteomics Introductory Lecture Fall 2012|User:Wayne Decatur/UNH BCHEM833 Structural Proteomics Introductory Lecture Fall 2012]] | *[[User:Wayne Decatur/UNH BCHEM833 Structural Proteomics Introductory Lecture Fall 2012|User:Wayne Decatur/UNH BCHEM833 Structural Proteomics Introductory Lecture Fall 2012]] | ||
*[[3sn6| | *[[3sn6|3SN6]] | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Latest revision as of 18:07, 25 February 2021
IntroductionG protein-coupled receptors (GPCRs) are a large family of protein receptors, which have seven-transmembrane helices and are found over a large array of eukaryotic cells. These receptors take a major part in a multitude of signal transduction pathways, including amongst others responses to hormones and neurotransmitters, sensing light, taste and smell, and many more. These receptors are also involved in many different types of diseases and are the target of almost 50% of current medical drugs. The Beta-2 Adrenergic Receptors are a type of GPCR that are activated by catecholamine hormone ligands such as adrenaline (epinephrine). These receptors are responsible for many of the adrenaline related (“fight-or-flight”) responses and functions, and are used as a common model system for the GPCR family. GPCRs bind their ligand and overcome a conformational change that activates an attached Guanine nucleotide-binding protein (G protein) and allows it to detach from the cellular end of the receptor and start the different signal transduction pathways. G proteins[1] are a family of proteins that act as molecular switches inside cells. G proteins belong to the larger group of enzymes called GTPases[2], and appear either as monomeric small GTPases[3], or as heterotrimeric G protein complexes[4] that are made up of alpha (α), beta (β) and gamma (γ) subunits[5]. When they are bound to guanosine triphosphate (GTP), they are 'on', and when they are bound to guanosine diphosphate (GDP), they are 'off'. Since these receptors have seven transmembrane helices as well as inner and outer cell regions, they are very difficult to purify and crystallize. Some crystal structures have been determined for the inactive receptors as well as for the G proteins that they bind. PDB entry 3SN6 is the first structure of the full complex of the Beta 2 Adrenergic Receptor bound to Gs in their active state, and it provides the first high-resolution insight into the mechanism of signal transduction across the plasma membrane by a GPCR. Complex structureThe overall structure shows the (dark blue) bound to an agonist (in spheres) along with a lysozyme fused to its amino terminus in order to facilitate crystallization. The receptor interacts with (light blue). Gαs together with (light green) and (gold) constitute the heterotrimeric G protein Gs. A Gs-binding nanobody , which also facilitates crystallization, binds the G protein between the α and β subunits.
G-Protein-GPCR InteractionsThe α5-helix of Gαs into a cavity formed on the intracellular side of the receptor by the opening of the transmembrane helices. Within the transmembrane core, the interactions are primarily non-polar - an exception involves of Tyr 391 of the α5-helix against Arg 131 of the conserved DRY sequence in TM3. Arg 131 also packs against Tyr 326 of the conserved NPxxY sequence in TM7. As the α5-helix exits the receptor it forms a network of polar interactions with TM5 and TM3. Receptor residues Thr 68 and Asp 130 interact with the ICL2 helix of the β2AR via Tyr 141, positioning the helix so that Phe 139 of the receptor a hydrophobic pocket on the G protein surface, thereby structurally linking receptor–G protein interactions with the highly conserved DRY motif of the β2AR.
G-Protein Activation CycleThe figure shows the G Protein cycle[6] - an extracellular agonist binding to the β2AR leads to of the cytoplasmic ends of transmembrane segments that enable the Gs heterotrimer to bind the receptor. GDP is released from the α subunit upon formation of β2AR–Gs complex. The GTP binds to the nucleotide-free α subunit resulting in dissociation of the α and βγ subunits from the receptor. The subunits regulate their respective effector proteins adenylyl cyclase (AC) and Ca2+ channels. The Gs heterotrimer reassembles from α and βγ subunits following hydrolysis of GTP to GDP in the α subunit.
G-Protein variabilityThe Gαs subunit consists of two domains, the and the . A previous structure of a GTPγS bound (i.e. active, "turned on") Gαs protein (PDB ID: 1AZT) showed that both domains are involved in nucleotide binding, as the nucleotide-binding pocket of the Gαs subunit is formed by the interface between GαsRas and GαsAH[7]. It was also previously known that the GsαAH domain has a variable position relative to the GsαRas domain between this GTP bound (active) state and the nucleotide free state[8][9][10][11]. However, the β2AR–Gs complex structure of the receptor attached to the empty (no guanosine phosphate attached) G protein enabled comparing it to the active (GTP bound) structure and by that showing - this is probably the most surprising observation arising from the β2AR–Gs complex.
See Also
|
| ||||||||||
ReferencesReferences
- ↑ https://en.wikipedia.org/wiki/G_protein
- ↑ https://en.wikipedia.org/wiki/GTPase
- ↑ https://en.wikipedia.org/wiki/Small_GTPase
- ↑ https://en.wikipedia.org/wiki/Heterotrimeric_G_protein
- ↑ Hurowitz EH, Melnyk JM, Chen YJ, Kouros-Mehr H, Simon MI, Shizuya H. Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res. 2000 Apr 28;7(2):111-20. doi: 10.1093/dnares/7.2.111. PMID:10819326 doi:http://dx.doi.org/10.1093/dnares/7.2.111
- ↑ Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011 Jul 19;477(7366):549-55. doi: 10.1038/nature10361. PMID:21772288 doi:10.1038/nature10361
- ↑ doi:10.1126/science.278.5345.1943
- ↑ Markby DW, Onrust R, Bourne HR. Separate GTP binding and GTPase activating domains of a G alpha subunit. Science. 1993 Dec 17;262(5141):1895-901. doi: 10.1126/science.8266082. PMID:8266082 doi:http://dx.doi.org/10.1126/science.8266082
- ↑ Van Eps N, Preininger AM, Alexander N, Kaya AI, Meier S, Meiler J, Hamm HE, Hubbell WL. Interaction of a G protein with an activated receptor opens the interdomain interface in the alpha subunit. Proc Natl Acad Sci U S A. 2011 Jun 7;108(23):9420-4. doi:, 10.1073/pnas.1105810108. Epub 2011 May 23. PMID:21606326 doi:http://dx.doi.org/10.1073/pnas.1105810108
- ↑ Westfield GH, Rasmussen SG, Su M, Dutta S, DeVree BT, Chung KY, Calinski D, Velez-Ruiz G, Oleskie AN, Pardon E, Chae PS, Liu T, Li S, Woods VL Jr, Steyaert J, Kobilka BK, Sunahara RK, Skiniotis G. Structural flexibility of the G alpha s alpha-helical domain in the beta2-adrenoceptor Gs complex. Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):16086-91. doi:, 10.1073/pnas.1113645108. Epub 2011 Sep 13. PMID:21914848 doi:http://dx.doi.org/10.1073/pnas.1113645108
- ↑ Chung KY, Rasmussen SG, Liu T, Li S, DeVree BT, Chae PS, Calinski D, Kobilka BK, Woods VL Jr, Sunahara RK. Conformational changes in the G protein Gs induced by the beta2 adrenergic receptor. Nature. 2011 Sep 28;477(7366):611-5. doi: 10.1038/nature10488. PMID:21956331 doi:10.1038/nature10488