1tyf: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==THE STRUCTURE OF CLPP AT 2.3 ANGSTROM RESOLUTION SUGGESTS A MODEL FOR ATP-DEPENDENT PROTEOLYSIS== | |||
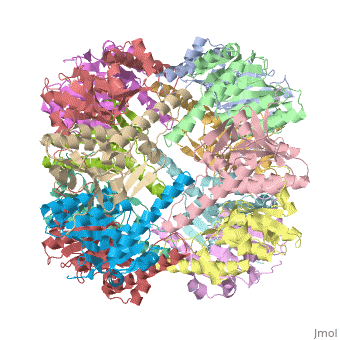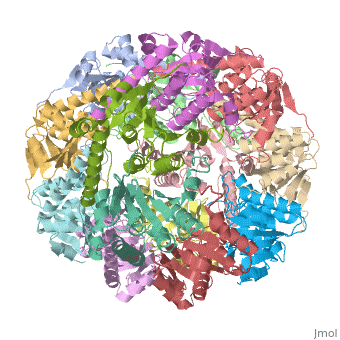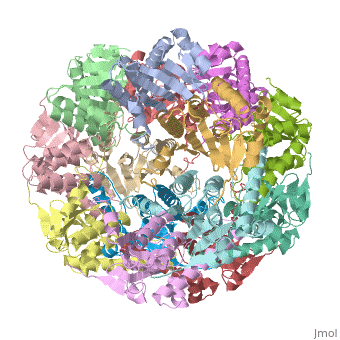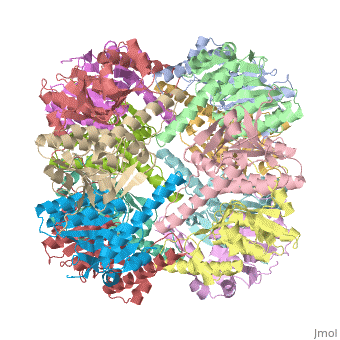
<StructureSection load='1tyf' size='340' side='right'caption='[[1tyf]], [[Resolution|resolution]] 2.30Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1tyf]] is a 14 chain structure with sequence from [https://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1TYF OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1TYF FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.3Å</td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1tyf FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1tyf OCA], [https://pdbe.org/1tyf PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1tyf RCSB], [https://www.ebi.ac.uk/pdbsum/1tyf PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1tyf ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/CLPP_ECOLI CLPP_ECOLI] Cleaves peptides in various proteins in a process that requires ATP hydrolysis. Has a chymotrypsin-like activity. Plays a major role in the degradation of misfolded proteins. May play the role of a master protease which is attracted to different substrates by different specificity factors such as ClpA or ClpX. | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
< | Check<jmol> | ||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/ty/1tyf_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
== | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1tyf ConSurf]. | ||
[[1tyf]] is a 14 chain structure with sequence from [ | <div style="clear:both"></div> | ||
==See Also== | ==See Also== | ||
*[[Clp protease|Clp protease]] | *[[Clp Protease|Clp Protease]] | ||
*[[Clp protease 3D structures|Clp protease 3D structures]] | |||
__TOC__ | |||
</StructureSection> | |||
[[Category: Escherichia coli]] | [[Category: Escherichia coli]] | ||
[[Category: | [[Category: Large Structures]] | ||
[[Category: | [[Category: Flanagan JM]] | ||
[[Category: | [[Category: Hartling JA]] | ||
[[Category: | [[Category: Wang J]] | ||
Latest revision as of 11:42, 14 February 2024
THE STRUCTURE OF CLPP AT 2.3 ANGSTROM RESOLUTION SUGGESTS A MODEL FOR ATP-DEPENDENT PROTEOLYSISTHE STRUCTURE OF CLPP AT 2.3 ANGSTROM RESOLUTION SUGGESTS A MODEL FOR ATP-DEPENDENT PROTEOLYSIS
Structural highlights
FunctionCLPP_ECOLI Cleaves peptides in various proteins in a process that requires ATP hydrolysis. Has a chymotrypsin-like activity. Plays a major role in the degradation of misfolded proteins. May play the role of a master protease which is attracted to different substrates by different specificity factors such as ClpA or ClpX. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. See Also |
| ||||||||||||||||