4awl: Difference between revisions
New page: '''Unreleased structure''' The entry 4awl is ON HOLD Authors: Nardini, M., Gnesutta, N., Donati, G., Gatta, R., Forni, C., Fossati, A., Vonrhein, C., Moras, D., Romier, C., Mantovani, R... |
No edit summary |
||
| (9 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
The | ==The NF-Y transcription factor is structurally and functionally a sequence specific histone== | ||
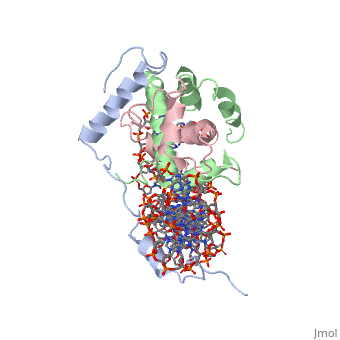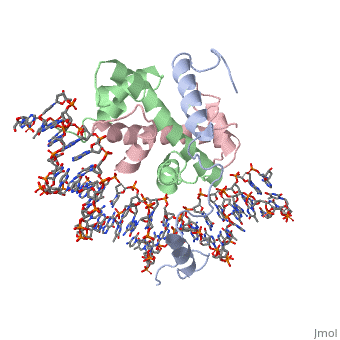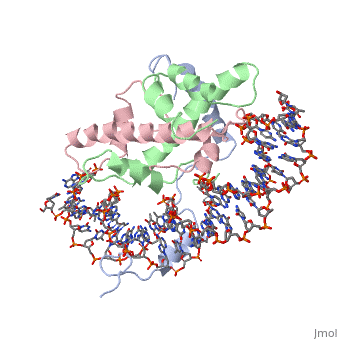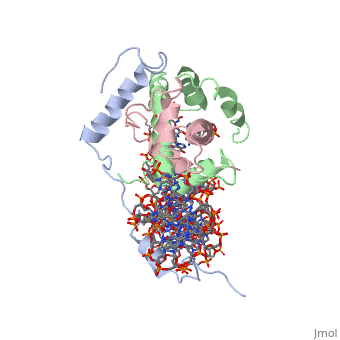
<StructureSection load='4awl' size='340' side='right'caption='[[4awl]], [[Resolution|resolution]] 3.08Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[4awl]] is a 5 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4AWL OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=4AWL FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.08Å</td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=4awl FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4awl OCA], [https://pdbe.org/4awl PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=4awl RCSB], [https://www.ebi.ac.uk/pdbsum/4awl PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=4awl ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/NFYA_HUMAN NFYA_HUMAN] Component of the sequence-specific heterotrimeric transcription factor (NF-Y) which specifically recognizes a 5'-CCAAT-3' box motif found in the promoters of its target genes. NF-Y can function as both an activator and a repressor, depending on its interacting cofactors. NF-YA positively regulates the transcription of the core clock component ARNTL/BMAL1.<ref>PMID:12741956</ref> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
The sequence-specific transcription factor NF-Y binds the CCAAT box, one of the sequence elements most frequently found in eukaryotic promoters. NF-Y is composed of the NF-YA and NF-YB/NF-YC subunits, the latter two hosting histone-fold domains (HFDs). The crystal structure of NF-Y bound to a 25 bp CCAAT oligonucleotide shows that the HFD dimer binds to the DNA sugar-phosphate backbone, mimicking the nucleosome H2A/H2B-DNA assembly. NF-YA both binds to NF-YB/NF-YC and inserts an alpha helix deeply into the DNA minor groove, providing sequence-specific contacts to the CCAAT box. Structural considerations and mutational data indicate that NF-YB ubiquitination at Lys138 precedes and is equivalent to H2B Lys120 monoubiquitination, important in transcriptional activation. Thus, NF-Y is a sequence-specific transcription factor with nucleosome-like properties of nonspecific DNA binding and helps establish permissive chromatin modifications at CCAAT promoters. Our findings suggest that other HFD-containing proteins may function in similar ways. | |||
Sequence-Specific Transcription Factor NF-Y Displays Histone-like DNA Binding and H2B-like Ubiquitination.,Nardini M, Gnesutta N, Donati G, Gatta R, Forni C, Fossati A, Vonrhein C, Moras D, Romier C, Bolognesi M, Mantovani R Cell. 2013 Jan 17;152(1-2):132-43. doi: 10.1016/j.cell.2012.11.047. PMID:23332751<ref>PMID:23332751</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 4awl" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Nuclear transcription factor Y|Nuclear transcription factor Y]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
[[Category: Homo sapiens]] | |||
[[Category: Large Structures]] | |||
[[Category: Bolognesi M]] | |||
[[Category: Donati G]] | |||
[[Category: Forni C]] | |||
[[Category: Fossati A]] | |||
[[Category: Gatta R]] | |||
[[Category: Gnesutta N]] | |||
[[Category: Mantovani R]] | |||
[[Category: Moras D]] | |||
[[Category: Nardini M]] | |||
[[Category: Romier C]] | |||
[[Category: Vonrhein C]] | |||
Latest revision as of 14:37, 20 December 2023
The NF-Y transcription factor is structurally and functionally a sequence specific histoneThe NF-Y transcription factor is structurally and functionally a sequence specific histone
Structural highlights
FunctionNFYA_HUMAN Component of the sequence-specific heterotrimeric transcription factor (NF-Y) which specifically recognizes a 5'-CCAAT-3' box motif found in the promoters of its target genes. NF-Y can function as both an activator and a repressor, depending on its interacting cofactors. NF-YA positively regulates the transcription of the core clock component ARNTL/BMAL1.[1] Publication Abstract from PubMedThe sequence-specific transcription factor NF-Y binds the CCAAT box, one of the sequence elements most frequently found in eukaryotic promoters. NF-Y is composed of the NF-YA and NF-YB/NF-YC subunits, the latter two hosting histone-fold domains (HFDs). The crystal structure of NF-Y bound to a 25 bp CCAAT oligonucleotide shows that the HFD dimer binds to the DNA sugar-phosphate backbone, mimicking the nucleosome H2A/H2B-DNA assembly. NF-YA both binds to NF-YB/NF-YC and inserts an alpha helix deeply into the DNA minor groove, providing sequence-specific contacts to the CCAAT box. Structural considerations and mutational data indicate that NF-YB ubiquitination at Lys138 precedes and is equivalent to H2B Lys120 monoubiquitination, important in transcriptional activation. Thus, NF-Y is a sequence-specific transcription factor with nucleosome-like properties of nonspecific DNA binding and helps establish permissive chromatin modifications at CCAAT promoters. Our findings suggest that other HFD-containing proteins may function in similar ways. Sequence-Specific Transcription Factor NF-Y Displays Histone-like DNA Binding and H2B-like Ubiquitination.,Nardini M, Gnesutta N, Donati G, Gatta R, Forni C, Fossati A, Vonrhein C, Moras D, Romier C, Bolognesi M, Mantovani R Cell. 2013 Jan 17;152(1-2):132-43. doi: 10.1016/j.cell.2012.11.047. PMID:23332751[2] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||