3ua0: Difference between revisions
No edit summary |
No edit summary |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
< | ==N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease== | ||
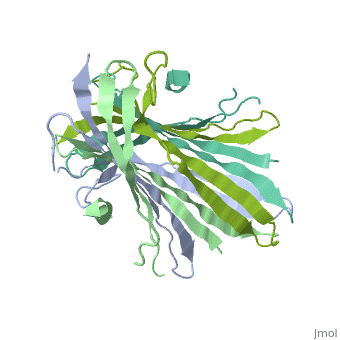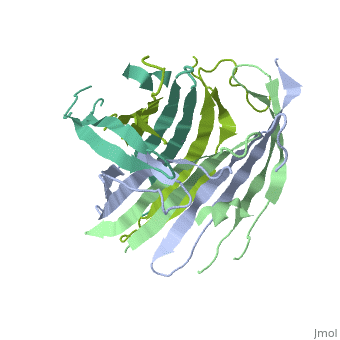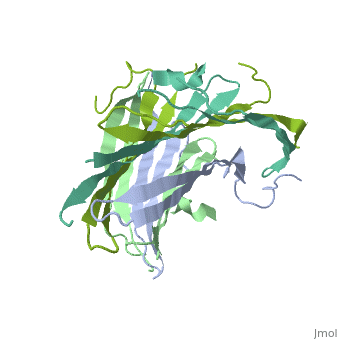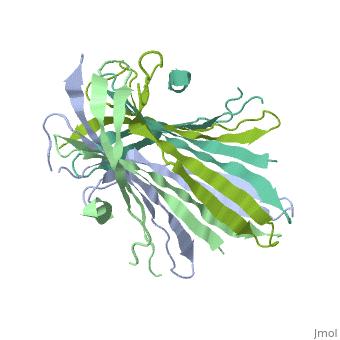
<StructureSection load='3ua0' size='340' side='right'caption='[[3ua0]], [[Resolution|resolution]] 3.00Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[3ua0]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Bombyx_mori Bombyx mori]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3UA0 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3UA0 FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3Å</td></tr> | |||
-- | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=MSE:SELENOMETHIONINE'>MSE</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3ua0 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3ua0 OCA], [https://pdbe.org/3ua0 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3ua0 RCSB], [https://www.ebi.ac.uk/pdbsum/3ua0 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3ua0 ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/FIBH_BOMMO FIBH_BOMMO] Core component of the silk filament; a strong, insoluble and chemically inert fiber. | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Fibroins serve as the major building blocks of silk fiber. As the major component of fibroin, the fibroin heavy chain is a considerably large protein comprising N-terminal and C-terminal hydrophilic domains and 12 highly repetitive Gly-Ala-rich regions flanked by internal hydrophilic blocks. Here, we show the crystal structure of the fibroin N-terminal domain (FibNT) at pH 4.7, revealing a remarkable double-layered anti-parallel beta-sheet with each layer comprising two FibNT molecules entangled together. We also show that FibNT undergoes a pH-responsive conformational transition from random coil to beta-sheets at around pH 6.0. Dynamic light scattering demonstrates that FibNT tends to oligomerize as pH decreases to 6.0, and electron microscopy reveals micelle-like oligomers. Our results are consistent with the micelle assembly model of silk fibroin and, more importantly, show that the N-terminal domain in itself has the capacity to form micelle-like structures in response to pH decrease. Structural and mutagenesis analyses further reveal the important role of conserved acidic residues clustered in FibNT, such as Glu56 and Asp100, in preventing premature beta-sheet formation at neutral pH. Collectively, we suggest that FibNT functions as a pH-responsive self-assembly module that could prevent premature beta-sheet formation at neutral pH yet could initiate fibroin assembly as pH decreases along the lumen of the posterior silk gland to the anterior silk gland. | |||
N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease.,He YX, Zhang NN, Li WF, Jia N, Chen BY, Zhou K, Zhang J, Chen Y, Zhou CZ J Mol Biol. 2012 Mar 1. PMID:22387468<ref>PMID:22387468</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 3ua0" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Fibroins|Fibroins]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
== | |||
[[ | |||
== | |||
< | |||
[[Category: Bombyx mori]] | [[Category: Bombyx mori]] | ||
[[Category: Chen | [[Category: Large Structures]] | ||
[[Category: Chen | [[Category: Chen B-Y]] | ||
[[Category: He | [[Category: Chen Y-X]] | ||
[[Category: Li | [[Category: He Y-X]] | ||
[[Category: Zhang | [[Category: Li W-F]] | ||
[[Category: Zhou | [[Category: Zhang N-N]] | ||
[[Category: Zhou C-Z]] | |||
Latest revision as of 13:31, 6 November 2024
N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH DecreaseN-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease
Structural highlights
FunctionFIBH_BOMMO Core component of the silk filament; a strong, insoluble and chemically inert fiber. Publication Abstract from PubMedFibroins serve as the major building blocks of silk fiber. As the major component of fibroin, the fibroin heavy chain is a considerably large protein comprising N-terminal and C-terminal hydrophilic domains and 12 highly repetitive Gly-Ala-rich regions flanked by internal hydrophilic blocks. Here, we show the crystal structure of the fibroin N-terminal domain (FibNT) at pH 4.7, revealing a remarkable double-layered anti-parallel beta-sheet with each layer comprising two FibNT molecules entangled together. We also show that FibNT undergoes a pH-responsive conformational transition from random coil to beta-sheets at around pH 6.0. Dynamic light scattering demonstrates that FibNT tends to oligomerize as pH decreases to 6.0, and electron microscopy reveals micelle-like oligomers. Our results are consistent with the micelle assembly model of silk fibroin and, more importantly, show that the N-terminal domain in itself has the capacity to form micelle-like structures in response to pH decrease. Structural and mutagenesis analyses further reveal the important role of conserved acidic residues clustered in FibNT, such as Glu56 and Asp100, in preventing premature beta-sheet formation at neutral pH. Collectively, we suggest that FibNT functions as a pH-responsive self-assembly module that could prevent premature beta-sheet formation at neutral pH yet could initiate fibroin assembly as pH decreases along the lumen of the posterior silk gland to the anterior silk gland. N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease.,He YX, Zhang NN, Li WF, Jia N, Chen BY, Zhou K, Zhang J, Chen Y, Zhou CZ J Mol Biol. 2012 Mar 1. PMID:22387468[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||