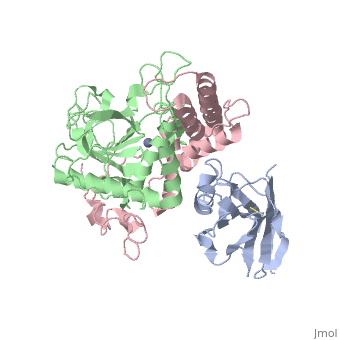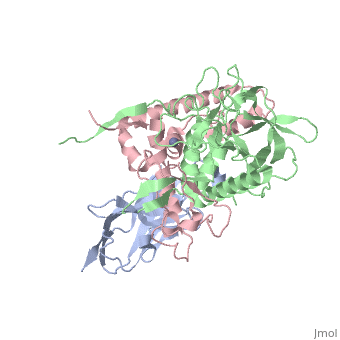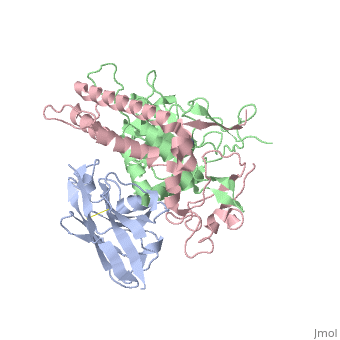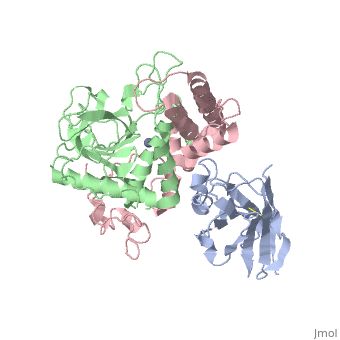3k3q: Difference between revisions
m Protected "3k3q" [edit=sysop:move=sysop] |
No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
< | ==Crystal Structure of a Llama Antibody complexed with the C. Botulinum Neurotoxin Serotype A Catalytic Domain== | ||
<StructureSection load='3k3q' size='340' side='right'caption='[[3k3q]], [[Resolution|resolution]] 2.60Å' scene=''> | |||
You may | == Structural highlights == | ||
or the | <table><tr><td colspan='2'>[[3k3q]] is a 3 chain structure with sequence from [https://en.wikipedia.org/wiki/Clostridium_botulinum_A_str._Hall Clostridium botulinum A str. Hall] and [https://en.wikipedia.org/wiki/Lama_glama Lama glama]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3K3Q OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3K3Q FirstGlance]. <br> | ||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.6Å</td></tr> | |||
-- | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3k3q FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3k3q OCA], [https://pdbe.org/3k3q PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3k3q RCSB], [https://www.ebi.ac.uk/pdbsum/3k3q PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3k3q ProSAT]</span></td></tr> | |||
</table> | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/k3/3k3q_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview03.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=3k3q ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Ingestion or inhalation of botulinum neurotoxin (BoNT) results in botulism, a severe and frequently fatal disease. Current treatments rely on antitoxins, which, while effective, cannot reverse symptoms once BoNT has entered the neuron. For treatments that can reverse intoxication, interest has focused on developing inhibitors of the enzymatic BoNT light chain (BoNT Lc). Such inhibitors typically mimic substrate and bind in or around the substrate cleavage pocket. To explore the full range of binding sites for serotype A light chain (BoNT/A Lc) inhibitors, we created a library of non-immune llama single-domain VHH (camelid heavy-chain variable region derived from heavy-chain-only antibody) antibodies displayed on the surface of the yeast Saccharomyces cerevisiae. Library selection on BoNT/A Lc yielded 15 yeast-displayed VHH with equilibrium dissociation constants (K(d)) from 230 to 0.03 nM measured by flow cytometry. Eight of 15 VHH inhibited the cleavage of substrate SNAP25 (synaptosome-associated protein of 25,000 Da) by BoNT/A Lc. The most potent VHH (Aa1) had a solution K(d) for BoNT/A Lc of 1.47 x 10(-)(10) M and an IC(50) (50% inhibitory concentration) of 4.7 x 10(-)(10) M and was resistant to heat denaturation and reducing conditions. To understand the mechanism by which Aa1 inhibited catalysis, we solved the X-ray crystal structure of the BoNT/A Lc-Aa1 VHH complex at 2.6 A resolution. The structure reveals that the Aa1 VHH binds in the alpha-exosite of the BoNT/A Lc, far from the active site for catalysis. The study validates the utility of non-immune llama VHH libraries as a source of enzyme inhibitors and identifies the BoNT/A Lc alpha-exosite as a target for inhibitor development. | |||
A single-domain llama antibody potently inhibits the enzymatic activity of botulinum neurotoxin by binding to the non-catalytic alpha-exosite binding region.,Dong J, Thompson AA, Fan Y, Lou J, Conrad F, Ho M, Pires-Alves M, Wilson BA, Stevens RC, Marks JD J Mol Biol. 2010 Apr 9;397(4):1106-18. Epub 2010 Feb 6. PMID:20138889<ref>PMID:20138889</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 3k3q" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Antibody 3D structures|Antibody 3D structures]] | |||
*[[Botulinum neurotoxin 3D structures|Botulinum neurotoxin 3D structures]] | |||
*[[3D structures of non-human antibody|3D structures of non-human antibody]] | |||
== References == | |||
<references/> | |||
== | __TOC__ | ||
[[ | </StructureSection> | ||
[[Category: Clostridium botulinum A str. Hall]] | |||
== | |||
< | |||
[[Category: Clostridium botulinum | |||
[[Category: Lama glama]] | [[Category: Lama glama]] | ||
[[Category: | [[Category: Large Structures]] | ||
[[Category: | [[Category: Dong J]] | ||
[[Category: | [[Category: Marks JD]] | ||
[[Category: | [[Category: Stevens RC]] | ||
[[Category: | [[Category: Thompson AA]] | ||
Latest revision as of 05:00, 21 November 2024
Crystal Structure of a Llama Antibody complexed with the C. Botulinum Neurotoxin Serotype A Catalytic DomainCrystal Structure of a Llama Antibody complexed with the C. Botulinum Neurotoxin Serotype A Catalytic Domain
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedIngestion or inhalation of botulinum neurotoxin (BoNT) results in botulism, a severe and frequently fatal disease. Current treatments rely on antitoxins, which, while effective, cannot reverse symptoms once BoNT has entered the neuron. For treatments that can reverse intoxication, interest has focused on developing inhibitors of the enzymatic BoNT light chain (BoNT Lc). Such inhibitors typically mimic substrate and bind in or around the substrate cleavage pocket. To explore the full range of binding sites for serotype A light chain (BoNT/A Lc) inhibitors, we created a library of non-immune llama single-domain VHH (camelid heavy-chain variable region derived from heavy-chain-only antibody) antibodies displayed on the surface of the yeast Saccharomyces cerevisiae. Library selection on BoNT/A Lc yielded 15 yeast-displayed VHH with equilibrium dissociation constants (K(d)) from 230 to 0.03 nM measured by flow cytometry. Eight of 15 VHH inhibited the cleavage of substrate SNAP25 (synaptosome-associated protein of 25,000 Da) by BoNT/A Lc. The most potent VHH (Aa1) had a solution K(d) for BoNT/A Lc of 1.47 x 10(-)(10) M and an IC(50) (50% inhibitory concentration) of 4.7 x 10(-)(10) M and was resistant to heat denaturation and reducing conditions. To understand the mechanism by which Aa1 inhibited catalysis, we solved the X-ray crystal structure of the BoNT/A Lc-Aa1 VHH complex at 2.6 A resolution. The structure reveals that the Aa1 VHH binds in the alpha-exosite of the BoNT/A Lc, far from the active site for catalysis. The study validates the utility of non-immune llama VHH libraries as a source of enzyme inhibitors and identifies the BoNT/A Lc alpha-exosite as a target for inhibitor development. A single-domain llama antibody potently inhibits the enzymatic activity of botulinum neurotoxin by binding to the non-catalytic alpha-exosite binding region.,Dong J, Thompson AA, Fan Y, Lou J, Conrad F, Ho M, Pires-Alves M, Wilson BA, Stevens RC, Marks JD J Mol Biol. 2010 Apr 9;397(4):1106-18. Epub 2010 Feb 6. PMID:20138889[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||