Jasper Small Lactate Sandbox 1: Difference between revisions
Jasper Small (talk | contribs) No edit summary |
Jasper Small (talk | contribs) No edit summary |
||
| (15 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Image:1i10.png|left|200px]] | [[Image:1i10.png|left|200px]] | ||
{{STRUCTURE_1i10| PDB=1i10 | SCENE= }} | {{STRUCTURE_1i10| PDB=1i10 | SCENE= }} | ||
| Line 14: | Line 6: | ||
==Lactate Dehydrogenase== | ==Lactate Dehydrogenase== | ||
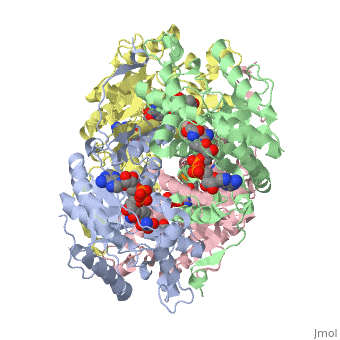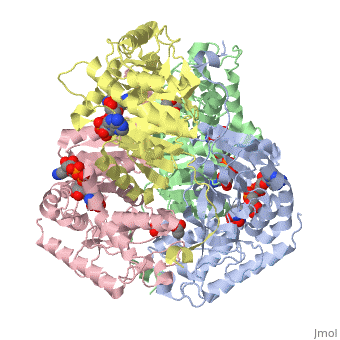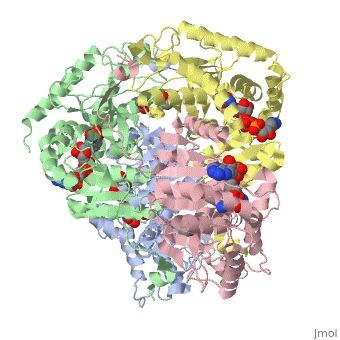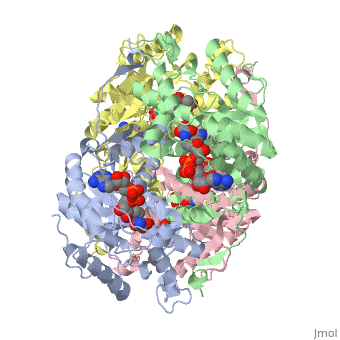
Lactate Dehydrogenase (LDH) is an important enzyme in humans. It occurs in different regions of the body, each region having a unique conformation of different subunits. The Jmole image shown is that of LDH-5, the form found in skeleton muscle and the liver. | <scene name='Jasper_Small_Lactate_Sandbox_1/Basic/1'>Lactate Dehydrogenase (LDH)</scene> is an important enzyme in humans. It occurs in different regions of the body, each region having a unique conformation of different subunits. The Jmole image shown is that of LDH-5, the form found in skeleton muscle and the liver. LDH is a key enzyme in anaerobic respiration. Anaerobic Respiration is the conversion of pyruvate into lactate acid in the absence oxygen. This pathway is important to glycolysis in two main ways. The first is that if pyruvate were to build up glycoysis and thus the generation of ATP would slow. The second is anaerobic respiration allows for the regeneration of NAD+ from NADH. NAD+ is required when glyceraldehyde-3-phosphate dehydrogenase oxidizes glyceraldehyde-3-phosphate in glycolysis, which generates NADH. Lactate dehydrogenase is responsible for the anaerobic conversion of NADH to NAD+. | ||
[[Image:LDH_reaction.jpeg|left|thumb|355px|Catalytic function of LDH (1)]] | |||
==Structure== | |||
LDH is a quaternary protein formed of the combination of two subunits, M and H (Muscle and Heart) into a structure of four of the subunits. The various combinations found in the human body are: | |||
*(4H) Heart | |||
*(3H1M) Reticuloendothelial | |||
*(2H2M) Lungs | |||
*(1H3M) Kidneys | |||
*(4M) Muscle and Liver | |||
The secondary structure of LDH is comprised of 40% alpha helices and 23% beta sheets.(2) | |||
SCOP | |||
1I10- a/b Mainly parallel beta sheets (beta-alpha-beta units) | |||
1I0Z- a/b Mainly parallel beta sheets (beta-alpha-beta units) | |||
==Catalysis== | |||
Studies have shown that the reaction mechanism of LDH follows an ordered sequence. In order for lactate to be oxidized NAD+ must bind to the enzyme first followed by lactate. Transfer of a hydride ion then happens quickly in either direction giving a mixture of the two teranary complexes, enzyme-NAD+-lactate and enzyme-NADH-pyruvate. Finally pyruvate dissociates from the enzyme followed by NADH. The rate limiting step in this reaction is the rate of dissociation of NADH. The same holds true in the reverse reaction that the coenzyme, NADH, must bind first before the substrate, pyruvate, can bind. The conversion of pyruvate to lactate with the subsequent regeneration of NAD+ is a very favorable(1). | |||
[[Image:2nd.png|left|thumb|355px|(1)]] | |||
[[Image: | Important Sites: | ||
*<scene name='Jasper_Small_Lactate_Sandbox_1/His_195/1'>HIS 195</scene> | |||
*<scene name='Jasper_Small_Lactate_Sandbox_1/Arg_109/1'>ARG 109</scene> | |||
*<scene name='Jasper_Small_Lactate_Sandbox_1/Asp_168/1'>ASP 168</scene> | |||
*<scene name='Jasper_Small_Lactate_Sandbox_1/Arg_171/1'>ARG 171</scene> | |||
==Reference== | ==Reference== | ||
1- http://www.bioc.aecom.yu.edu/labs/calllab/highlights/LDH.htm | |||
2- http://www.cheric.org/ippage/e/ipdata/2004/05/file/e200405-701.pdf | |||
Latest revision as of 14:16, 5 April 2010
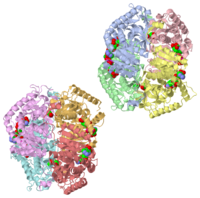
HUMAN MUSCLE L-LACTATE DEHYDROGENASE M CHAIN, TERNARY COMPLEX WITH NADH AND OXAMATEHUMAN MUSCLE L-LACTATE DEHYDROGENASE M CHAIN, TERNARY COMPLEX WITH NADH AND OXAMATE
Lactate DehydrogenaseLactate Dehydrogenase
is an important enzyme in humans. It occurs in different regions of the body, each region having a unique conformation of different subunits. The Jmole image shown is that of LDH-5, the form found in skeleton muscle and the liver. LDH is a key enzyme in anaerobic respiration. Anaerobic Respiration is the conversion of pyruvate into lactate acid in the absence oxygen. This pathway is important to glycolysis in two main ways. The first is that if pyruvate were to build up glycoysis and thus the generation of ATP would slow. The second is anaerobic respiration allows for the regeneration of NAD+ from NADH. NAD+ is required when glyceraldehyde-3-phosphate dehydrogenase oxidizes glyceraldehyde-3-phosphate in glycolysis, which generates NADH. Lactate dehydrogenase is responsible for the anaerobic conversion of NADH to NAD+.

StructureStructure
LDH is a quaternary protein formed of the combination of two subunits, M and H (Muscle and Heart) into a structure of four of the subunits. The various combinations found in the human body are:
- (4H) Heart
- (3H1M) Reticuloendothelial
- (2H2M) Lungs
- (1H3M) Kidneys
- (4M) Muscle and Liver
The secondary structure of LDH is comprised of 40% alpha helices and 23% beta sheets.(2) SCOP 1I10- a/b Mainly parallel beta sheets (beta-alpha-beta units) 1I0Z- a/b Mainly parallel beta sheets (beta-alpha-beta units)
CatalysisCatalysis
Studies have shown that the reaction mechanism of LDH follows an ordered sequence. In order for lactate to be oxidized NAD+ must bind to the enzyme first followed by lactate. Transfer of a hydride ion then happens quickly in either direction giving a mixture of the two teranary complexes, enzyme-NAD+-lactate and enzyme-NADH-pyruvate. Finally pyruvate dissociates from the enzyme followed by NADH. The rate limiting step in this reaction is the rate of dissociation of NADH. The same holds true in the reverse reaction that the coenzyme, NADH, must bind first before the substrate, pyruvate, can bind. The conversion of pyruvate to lactate with the subsequent regeneration of NAD+ is a very favorable(1).

Important Sites:
ReferenceReference
1- http://www.bioc.aecom.yu.edu/labs/calllab/highlights/LDH.htm 2- http://www.cheric.org/ippage/e/ipdata/2004/05/file/e200405-701.pdf