Sentrin-specific protease: Difference between revisions
Jump to navigation
Jump to search
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
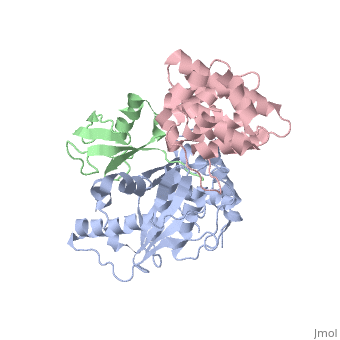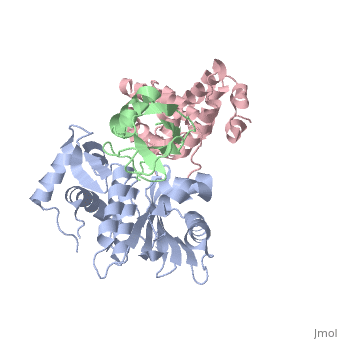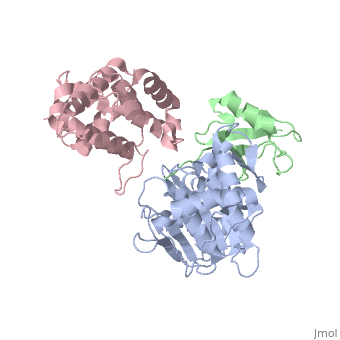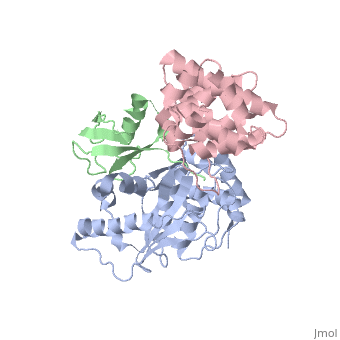
<StructureSection load='2iy0' size='350' side='right' caption='Structure of human Senp1 catalytic domain (grey) complex with SUMO1 (green) and RanGAP C terminal (pink) (PDB entry [[2iy0]])' scene=''> | <StructureSection load='2iy0' size='350' side='right' caption='Structure of human Senp1 catalytic domain (grey) complex with SUMO1 (green) and RanGAP C terminal (pink) (PDB entry [[2iy0]])' scene='' pspeed='8'> | ||
'''Sentrin-specific protease''' 1 (Senp1), Senp2, Senp7, Senp8 catalyze the processing of SUM1, SUMO2 and SUMO3 to their mature forms and deconjugate these proteins from their target proteins. Senp’s hydrolyze a peptide bond in the C-terminal of SUMO propeptide<ref>PMID:24586832</ref>. | '''Sentrin-specific protease''' 1 (Senp1), Senp2, Senp7, Senp8 catalyze the processing of SUM1, SUMO2 and SUMO3 to their mature forms and deconjugate these proteins from their target proteins. Senp’s hydrolyze a peptide bond in the C-terminal of SUMO propeptide<ref>PMID:24586832</ref>.<br /> | ||
*'''SENP2''' localised in cytoplasmic vesicles and nuclear body. | |||
*'''SENP7''' localised in breast epithelium. | |||
<scene name='56/568937/Cv/1'>human Senp1 catalytic domain complex with SUMO1 and RanGAP C terminal</scene> (PDB entry [[2iy0]]). | |||
</StructureSection> | </StructureSection> | ||
| Line 12: | Line 16: | ||
*Sentrin-specific protease 1 | *Sentrin-specific protease 1 | ||
**[[2ckg]], [[2iyc]] – hSenp1 | **[[2ckg]], [[2iyc]] – hSenp1 catalytic domain – human<br /> | ||
**[[2xph]], [[2xre]] – hSenp1 | **[[2xph]], [[2xre]] – hSenp1 catalytic domain + Co<br /> | ||
**[[ | **[[2iy1]], [[2g4d]] – hSenp1 catalytic domain (mutant) + SUMO1<br /> | ||
**[[ | **[[2ckh]], [[2iyd]] – hSenp1 catalytic domain + SUMO2<br /> | ||
**[[2iy0]] – hSenp1 | **[[6nnq]] – hSenp1 catalytic domain + SUMO3<br /> | ||
**[[2iy0]] – hSenp1 catalytic domain + SUMO1 + RanGAP C terminal<br /> | |||
*Sentrin-specific protease 2 | *Sentrin-specific protease 2 | ||
**[[1th0]] – hSenp2 catalytic domain <br /> | |||
**[[1tgz]] – hSenp2 catalytic domain + SUMO1<br /> | **[[1tgz]] – hSenp2 catalytic domain + SUMO1<br /> | ||
**[[2io0]], [[5aek]] – hSenp2 catalytic domain (mutant) + SUMO1 precursor<br /> | **[[2io0]], [[5aek]] – hSenp2 catalytic domain (mutant) + SUMO1 precursor<br /> | ||
**[[3zo5]] - hSenp2 catalytic domain (mutant) + SUMO2 precursor<br /> | **[[3zo5]] - hSenp2 catalytic domain (mutant) + SUMO2 precursor<br /> | ||
| Line 31: | Line 36: | ||
**[[3eay]] – hSenp7 catalytic domain + SUMO1<br /> | **[[3eay]] – hSenp7 catalytic domain + SUMO1<br /> | ||
**[[7r2e]] – hSenp7 catalytic domain + SUMO2<br /> | |||
*Sentrin-specific protease 8 | *Sentrin-specific protease 8 | ||
Latest revision as of 12:44, 11 August 2024
Sentrin-specific protease 1 (Senp1), Senp2, Senp7, Senp8 catalyze the processing of SUM1, SUMO2 and SUMO3 to their mature forms and deconjugate these proteins from their target proteins. Senp’s hydrolyze a peptide bond in the C-terminal of SUMO propeptide[1].
(PDB entry 2iy0). |
| ||||||||||
3D structures of sentrin-specific protease3D structures of sentrin-specific protease
Updated on 11-August-2024
ReferencesReferences
- ↑ Lee WP, Jena S, Doherty D, Ventakesh J, Schimdt J, Furmick J, Widener T, Lemau J, Jurutka PW, Thompson PD. Sentrin/SUMO specific proteases as novel tissue-selective modulators of vitamin D receptor-mediated signaling. PLoS One. 2014 Feb 20;9(2):e89506. doi: 10.1371/journal.pone.0089506. eCollection, 2014. PMID:24586832 doi:http://dx.doi.org/10.1371/journal.pone.0089506