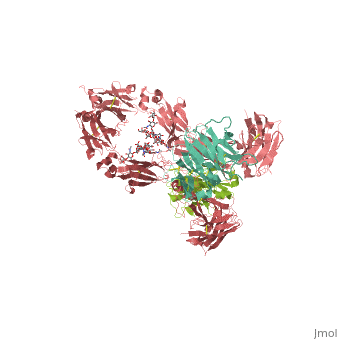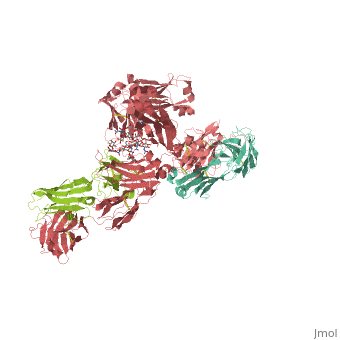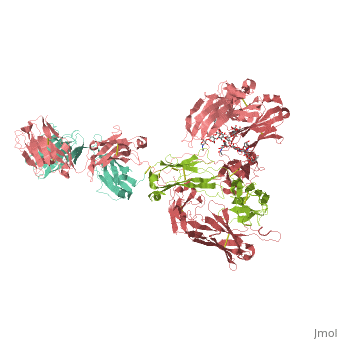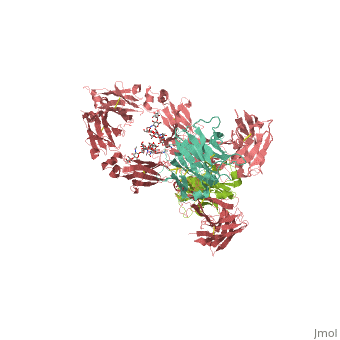1hzh: Difference between revisions
No edit summary |
No edit summary |
||
| (10 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== | ==CRYSTAL STRUCTURE OF THE INTACT HUMAN IGG B12 WITH BROAD AND POTENT ACTIVITY AGAINST PRIMARY HIV-1 ISOLATES: A TEMPLATE FOR HIV VACCINE DESIGN== | ||
[[1hzh]] is a 4 chain structure with sequence from [ | <StructureSection load='1hzh' size='340' side='right'caption='[[1hzh]], [[Resolution|resolution]] 2.70Å' scene=''> | ||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1hzh]] is a 4 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. The September 2001 RCSB PDB [https://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''Antibodies'' by David S. Goodsell is [https://dx.doi.org/10.2210/rcsb_pdb/mom_2001_9 10.2210/rcsb_pdb/mom_2001_9]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1HZH OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1HZH FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.7Å</td></tr> | |||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=BMA:BETA-D-MANNOSE'>BMA</scene>, <scene name='pdbligand=FUC:ALPHA-L-FUCOSE'>FUC</scene>, <scene name='pdbligand=GAL:BETA-D-GALACTOSE'>GAL</scene>, <scene name='pdbligand=MAN:ALPHA-D-MANNOSE'>MAN</scene>, <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1hzh FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1hzh OCA], [https://pdbe.org/1hzh PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1hzh RCSB], [https://www.ebi.ac.uk/pdbsum/1hzh PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1hzh ProSAT]</span></td></tr> | |||
</table> | |||
== Disease == | |||
[https://www.uniprot.org/uniprot/IGHG1_HUMAN IGHG1_HUMAN] Defects in IGHG1 are a cause of multiple myeloma (MM) [MIM:[https://omim.org/entry/254500 254500]. MM is a malignant tumor of plasma cells usually arising in the bone marrow and characterized by diffuse involvement of the skeletal system, hyperglobulinemia, Bence-Jones proteinuria and anemia. Complications of multiple myeloma are bone pain, hypercalcemia, renal failure and spinal cord compression. The aberrant antibodies that are produced lead to impaired humoral immunity and patients have a high prevalence of infection. Amyloidosis may develop in some patients. Multiple myeloma is part of a spectrum of diseases ranging from monoclonal gammopathy of unknown significance (MGUS) to plasma cell leukemia. Note=A chromosomal aberration involving IGHG1 is found in multiple myeloma. Translocation t(11;14)(q13;q32) with the IgH locus. Translocation t(11;14)(q13;q32) with CCND1; translocation t(4;14)(p16.3;q32.3) with FGFR3; translocation t(6;14)(p25;q32) with IRF4. | |||
== Function == | |||
[https://www.uniprot.org/uniprot/IGHG1_HUMAN IGHG1_HUMAN] | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/hz/1hzh_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview03.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1hzh ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
We present the crystal structure at 2.7 angstrom resolution of the human antibody IgG1 b12. Antibody b12 recognizes the CD4-binding site of human immunodeficiency virus-1 (HIV-1) gp120 and is one of only two known antibodies against gp120 capable of broad and potent neutralization of primary HIV-1 isolates. A key feature of the antibody-combining site is the protruding, finger-like long CDR H3 that can penetrate the recessed CD4-binding site of gp120. A docking model of b12 and gp120 reveals severe structural constraints that explain the extraordinary challenge in eliciting effective neutralizing antibodies similar to b12. The structure, together with mutagenesis studies, provides a rationale for the extensive cross-reactivity of b12 and a valuable framework for the design of HIV-1 vaccines capable of eliciting b12-like activity. | |||
Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design.,Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, Dwek RA, Stanfield RL, Burton DR, Wilson IA Science. 2001 Aug 10;293(5532):1155-9. PMID:11498595<ref>PMID:11498595</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 1hzh" style="background-color:#fffaf0;"></div> | |||
==See Also== | ==See Also== | ||
*[[Antibody|Antibody]] | *[[Antibody|Antibody]] | ||
*[[ | *[[Antibody 3D structures|Antibody 3D structures]] | ||
*[[ | *[[3D structures of human antibody|3D structures of human antibody]] | ||
== References == | |||
<references/> | |||
== | __TOC__ | ||
< | </StructureSection> | ||
[[Category: Antibodies]] | [[Category: Antibodies]] | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Large Structures]] | |||
[[Category: RCSB PDB Molecule of the Month]] | [[Category: RCSB PDB Molecule of the Month]] | ||
[[Category: Burton | [[Category: Burton DR]] | ||
[[Category: Saphire | [[Category: Saphire EO]] | ||
[[Category: Wilson | [[Category: Wilson IA]] | ||
Latest revision as of 03:04, 21 November 2024
CRYSTAL STRUCTURE OF THE INTACT HUMAN IGG B12 WITH BROAD AND POTENT ACTIVITY AGAINST PRIMARY HIV-1 ISOLATES: A TEMPLATE FOR HIV VACCINE DESIGNCRYSTAL STRUCTURE OF THE INTACT HUMAN IGG B12 WITH BROAD AND POTENT ACTIVITY AGAINST PRIMARY HIV-1 ISOLATES: A TEMPLATE FOR HIV VACCINE DESIGN
Structural highlights
DiseaseIGHG1_HUMAN Defects in IGHG1 are a cause of multiple myeloma (MM) [MIM:254500. MM is a malignant tumor of plasma cells usually arising in the bone marrow and characterized by diffuse involvement of the skeletal system, hyperglobulinemia, Bence-Jones proteinuria and anemia. Complications of multiple myeloma are bone pain, hypercalcemia, renal failure and spinal cord compression. The aberrant antibodies that are produced lead to impaired humoral immunity and patients have a high prevalence of infection. Amyloidosis may develop in some patients. Multiple myeloma is part of a spectrum of diseases ranging from monoclonal gammopathy of unknown significance (MGUS) to plasma cell leukemia. Note=A chromosomal aberration involving IGHG1 is found in multiple myeloma. Translocation t(11;14)(q13;q32) with the IgH locus. Translocation t(11;14)(q13;q32) with CCND1; translocation t(4;14)(p16.3;q32.3) with FGFR3; translocation t(6;14)(p25;q32) with IRF4. FunctionEvolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedWe present the crystal structure at 2.7 angstrom resolution of the human antibody IgG1 b12. Antibody b12 recognizes the CD4-binding site of human immunodeficiency virus-1 (HIV-1) gp120 and is one of only two known antibodies against gp120 capable of broad and potent neutralization of primary HIV-1 isolates. A key feature of the antibody-combining site is the protruding, finger-like long CDR H3 that can penetrate the recessed CD4-binding site of gp120. A docking model of b12 and gp120 reveals severe structural constraints that explain the extraordinary challenge in eliciting effective neutralizing antibodies similar to b12. The structure, together with mutagenesis studies, provides a rationale for the extensive cross-reactivity of b12 and a valuable framework for the design of HIV-1 vaccines capable of eliciting b12-like activity. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design.,Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, Dwek RA, Stanfield RL, Burton DR, Wilson IA Science. 2001 Aug 10;293(5532):1155-9. PMID:11498595[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||