Dihydropteroate synthase: Difference between revisions
No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (26 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
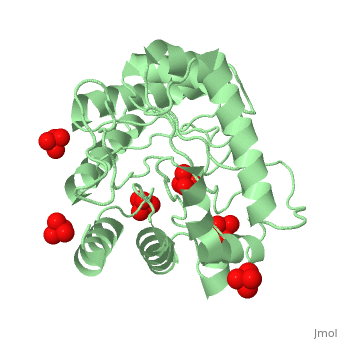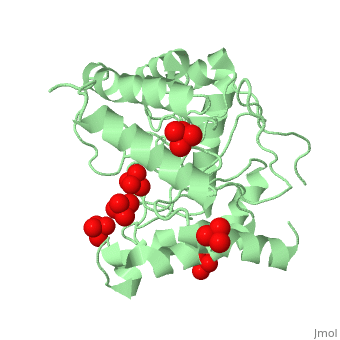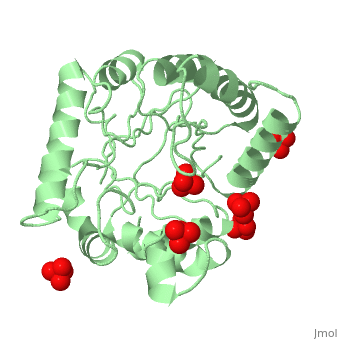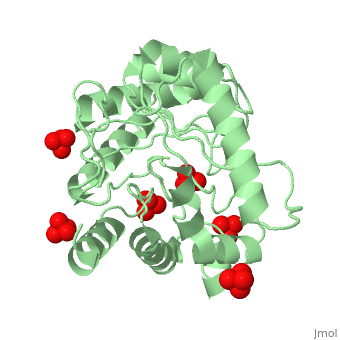
<StructureSection load='1tws' size='350' side='right' scene='' caption='Dihydropteroate synthase complex with sulfate (PDB code [[1tws]])'> | |||
__TOC__ | |||
==Function== | |||
'''DHPS | '''Dihydropteroate synthase''' (DHPS) catalyzes the condensation of 6-hydroxymethyl-7,8-dihydropteridine pyrophosphate to para-aminobenzoic acid (PABA) to form 7,8-dihydropteroate. DHPs is a key enzyme in folate synthesis. Folate is necessary for nucleic acid synthesis. DHPS is found in bacteria and not in eukaryotes. Hence, it makes a target to sulfonamide antibiotics<ref>PMID:10329458</ref> | ||
*'''7,8-dihydro-6-hydroxymethylpterin pyrophsphokinase-DHPS''' contains a dihydro-6-hydroxymethylpterin pyrophosphokinase domain at the N terminal and is named '''HPPK-DHPS'''. | |||
== Insights into the drug resistance induced by the BaDHPS mutations: molecular dynamic simulations and MM/GBSA studies <ref>doi 10.1080/07391102.2012.726529</ref>== | |||
''' | Drug resistance has been an urgent problem that severely limits the therapy of current clinical microbial diseases. Sometimes, it generally correlates with mutations to the dihydropteroate synthase (DHPS) gene. | ||
In the current study, we focus on the molecular dynamic behaviors and binding free energy calculations of <scene name='50/509381/Cv/10'>wild-type (wt)</scene> form and <scene name='50/509381/Cv/11'>mutated forms</scene> ''B. anthracis'' dihydropteroate synthase (BaDHPS) to search for the relationship between mutation and drug resistance. <span style="color:khaki;background-color:black;font-weight:bold;">Wt-BaDHPS is colored in khaki</span>, mutated <span style="color:lime;background-color:black;font-weight:bold;">D184N complex is in green</span> and <span style="color:cyan;background-color:black;font-weight:bold;">K220Q complex is in cyan</span>. | |||
After 20ns MD simulations on the <scene name='50/509381/Cv/12'>wt form and mutated form enzymes</scene>, it is obvious that <scene name='50/509381/8/1'>mutation D184N and K220Q have much lower binding affinity to the inhibitor DHP-STZ than the wt form enzyme</scene>. Only Loop 1, Loop 2 and Loop 7 are colored, ligand DHP-STZ is colored in the same color as the corresponding protein: for <span style="color:khaki;background-color:black;font-weight:bold;">Wt-BaDHPS is colored in khaki</span>, for mutated <span style="color:lime;background-color:black;font-weight:bold;">D184N complex is in green</span> and for <span style="color:cyan;background-color:black;font-weight:bold;">K220Q complex is in cyan</span>. Mutation will cause conformational change, which mainly locate on some loop region around the binding site (Loop 1, Loop 2 and Loop 7). These results may be helpful for further drug resistance and de novo drug design investigations. | |||
==3D structures of dihydropteroate synthase== | |||
[[Dihydropteroate synthase 3D structures]] | |||
[[ | |||
</StructureSection> | |||
==References== | |||
<references/> | |||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Latest revision as of 11:49, 13 June 2024
FunctionDihydropteroate synthase (DHPS) catalyzes the condensation of 6-hydroxymethyl-7,8-dihydropteridine pyrophosphate to para-aminobenzoic acid (PABA) to form 7,8-dihydropteroate. DHPs is a key enzyme in folate synthesis. Folate is necessary for nucleic acid synthesis. DHPS is found in bacteria and not in eukaryotes. Hence, it makes a target to sulfonamide antibiotics[1]
Insights into the drug resistance induced by the BaDHPS mutations: molecular dynamic simulations and MM/GBSA studies [2]Drug resistance has been an urgent problem that severely limits the therapy of current clinical microbial diseases. Sometimes, it generally correlates with mutations to the dihydropteroate synthase (DHPS) gene. In the current study, we focus on the molecular dynamic behaviors and binding free energy calculations of form and B. anthracis dihydropteroate synthase (BaDHPS) to search for the relationship between mutation and drug resistance. Wt-BaDHPS is colored in khaki, mutated D184N complex is in green and K220Q complex is in cyan. After 20ns MD simulations on the , it is obvious that . Only Loop 1, Loop 2 and Loop 7 are colored, ligand DHP-STZ is colored in the same color as the corresponding protein: for Wt-BaDHPS is colored in khaki, for mutated D184N complex is in green and for K220Q complex is in cyan. Mutation will cause conformational change, which mainly locate on some loop region around the binding site (Loop 1, Loop 2 and Loop 7). These results may be helpful for further drug resistance and de novo drug design investigations. 3D structures of dihydropteroate synthaseDihydropteroate synthase 3D structures
|
| ||||||||||
ReferencesReferences
- ↑ Vinnicombe HG, Derrick JP. Dihydropteroate synthase from Streptococcus pneumoniae: characterization of substrate binding order and sulfonamide inhibition. Biochem Biophys Res Commun. 1999 May 19;258(3):752-7. doi:, 10.1006/bbrc.1999.0695. PMID:10329458 doi:http://dx.doi.org/10.1006/bbrc.1999.0695
- ↑ Chu WT, Zhang JL, Zheng QC, Chen L, Xue Q, Zhang HX. Insights into the drug resistance induced by the BaDHPS mutations: molecular dynamic simulations and MM/GBSA studies. J Biomol Struct Dyn. 2012 Oct 2. PMID:23030549 doi:10.1080/07391102.2012.726529