Ferritin: Difference between revisions
Jump to navigation
Jump to search
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
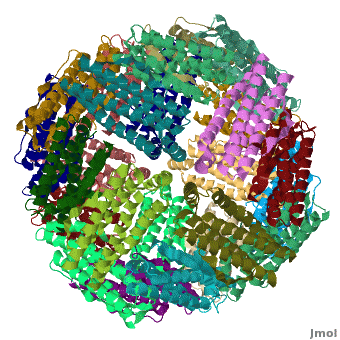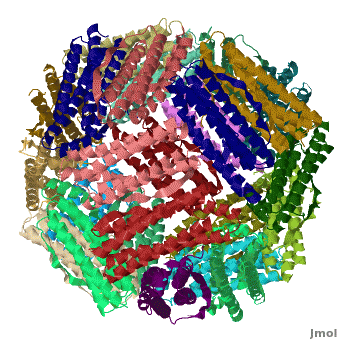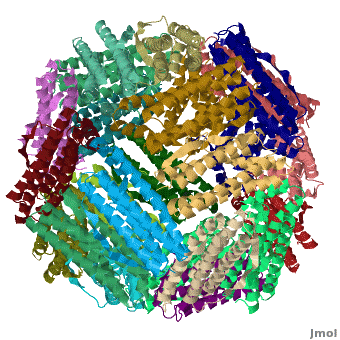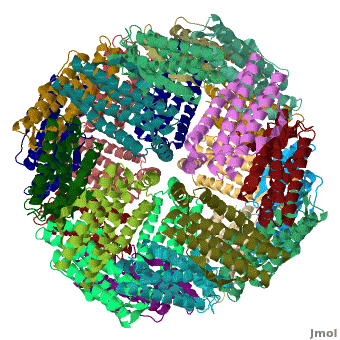
[[Ferritin]] (FR) is an iron storage and release protein. It stores iron as microcrystals with phosphate and hydroxide ions. FR is composed of 24 subunits of heavy chain (FTH) and light chain (FTL).<ref>PMID:20304033</ref> Amphibians have an additional middle subunit FR (FTM).<br /> | [[Ferritin]] (FR) is an iron storage and release protein. It stores iron as microcrystals with phosphate and hydroxide ions. FR is composed of 24 subunits of heavy chain (FTH) and light chain (FTL).<ref>PMID:20304033</ref> Amphibians have an additional middle subunit FR (FTM).<br /> | ||
* '''apo-ferritin''' (apo-FR) is a non-Fe-containing ferritin.<br /> | * '''apo-ferritin''' (apo-FR) is a non-Fe-containing ferritin.<br /> | ||
* '''Bacterioferritin''' (BFR) structure is very similar to FR. It contains a binuclear iron center and haem. It stores iron as ferric oxide mineral in its hollow central cavity.<br /> | * '''Bacterioferritin''' (BFR) structure is very similar to FR. It contains a binuclear iron center and haem. It stores iron as ferric oxide mineral in its hollow central cavity<ref>PMID:37375226</ref>.<br /> | ||
* '''Thioferritin''' (TFR) is a ferritin-related protein with 2 cysteine residues adjacent to the dimetal binding site.<br /> | * '''Thioferritin''' (TFR) is a ferritin-related protein with 2 cysteine residues adjacent to the dimetal binding site.<br /> | ||
* | * '''DNA-binding Protein of Starved cells''' (Dps) - a ferritin-like diiron carboxylate which acts as a molecular chaperone and may confer thermotolerance in ''E. coli''<ref>PMID:37229826</ref>.<br /> | ||
== Relevance == | == Relevance == | ||
Latest revision as of 09:35, 23 June 2024
FunctionFerritin (FR) is an iron storage and release protein. It stores iron as microcrystals with phosphate and hydroxide ions. FR is composed of 24 subunits of heavy chain (FTH) and light chain (FTL).[1] Amphibians have an additional middle subunit FR (FTM).
RelevanceCavities formed by FR are used for the manufacture of nanoparticles. FR is used as a marker for iron overload disorder. DiseaseFR deficiency can lead to anemia. 3D Structures of Ferritin
|
| ||||||||||
ReferencesReferences
- ↑ Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochim Biophys Acta. 2010 Aug;1800(8):760-9. doi: 10.1016/j.bbagen.2010.03.011. , Epub 2010 Mar 19. PMID:20304033 doi:http://dx.doi.org/10.1016/j.bbagen.2010.03.011
- ↑ van der Ven AM, Gyamfi H, Suttisansanee U, Ahmad MS, Su Z, Taylor RM, Poole A, Chiorean S, Daub E, Urquhart T, Honek JF. Molecular Engineering of E. coli Bacterioferritin: A Versatile Nanodimensional Protein Cage. Molecules. 2023 Jun 9;28(12):4663. PMID:37375226 doi:10.3390/molecules28124663
- ↑ Park JH, Lee ES, Jung YJ. Functional characterization of the DNA-binding protein from starved cells (DPS) as a molecular chaperone under heat stress. Biochem Biophys Res Commun. 2023 Jul 30;667:180-185. PMID:37229826 doi:10.1016/j.bbrc.2023.05.064