3mmt: Difference between revisions
No edit summary |
No edit summary |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
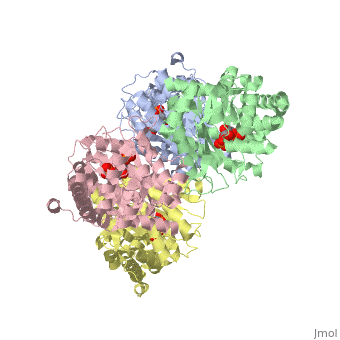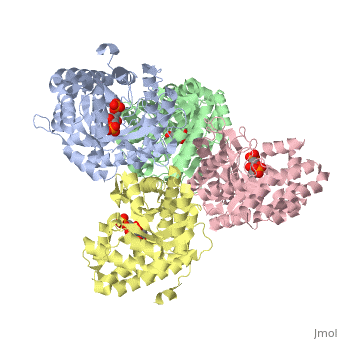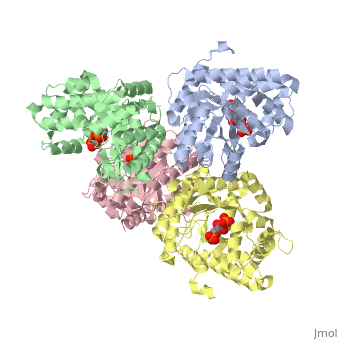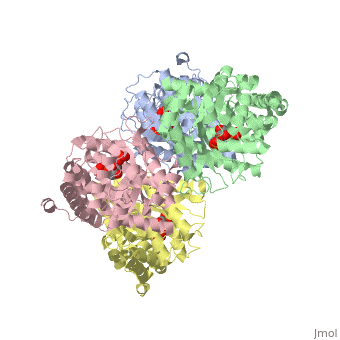
==Crystal structure of fructose bisphosphate aldolase from Bartonella henselae, bound to fructose bisphosphate== | ==Crystal structure of fructose bisphosphate aldolase from Bartonella henselae, bound to fructose bisphosphate== | ||
<StructureSection load='3mmt' size='340' side='right' caption='[[3mmt]], [[Resolution|resolution]] 2.35Å' scene=''> | <StructureSection load='3mmt' size='340' side='right'caption='[[3mmt]], [[Resolution|resolution]] 2.35Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[3mmt]] is a 4 chain structure with sequence from [ | <table><tr><td colspan='2'>[[3mmt]] is a 4 chain structure with sequence from [https://en.wikipedia.org/wiki/Bartonella_henselae Bartonella henselae]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3MMT OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3MMT FirstGlance]. <br> | ||
</td></tr><tr id=' | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.35Å</td></tr> | ||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=2FP:1,6-FRUCTOSE+DIPHOSPHATE+(LINEAR+FORM)'>2FP</scene></td></tr> | |||
<tr id=' | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3mmt FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3mmt OCA], [https://pdbe.org/3mmt PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3mmt RCSB], [https://www.ebi.ac.uk/pdbsum/3mmt PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3mmt ProSAT]</span></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | |||
</table> | </table> | ||
== Function == | |||
[https://www.uniprot.org/uniprot/Q8L207_BARHN Q8L207_BARHN] | |||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
Check<jmol> | Check<jmol> | ||
<jmolCheckbox> | <jmolCheckbox> | ||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/mm/3mmt_consurf.spt"</scriptWhenChecked> | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/mm/3mmt_consurf.spt"</scriptWhenChecked> | ||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/ | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview03.spt</scriptWhenUnchecked> | ||
<text>to colour the structure by Evolutionary Conservation</text> | <text>to colour the structure by Evolutionary Conservation</text> | ||
</jmolCheckbox> | </jmolCheckbox> | ||
| Line 32: | Line 32: | ||
==See Also== | ==See Also== | ||
*[[Aldolase|Aldolase]] | *[[Aldolase|Aldolase]] | ||
*[[Aldolase 3D structures|Aldolase 3D structures]] | |||
== References == | == References == | ||
<references/> | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: | [[Category: Bartonella henselae]] | ||
[[Category: | [[Category: Large Structures]] | ||
Latest revision as of 05:08, 21 November 2024
Crystal structure of fructose bisphosphate aldolase from Bartonella henselae, bound to fructose bisphosphateCrystal structure of fructose bisphosphate aldolase from Bartonella henselae, bound to fructose bisphosphate
Structural highlights
FunctionEvolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedFructose bisphosphate aldolase (FBPA) enzymes have been found in a broad range of eukaryotic and prokaryotic organisms. FBPA catalyses the cleavage of fructose 1,6-bisphosphate into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate. The SSGCID has reported several FBPA structures from pathogenic sources, including the bacterium Brucella melitensis and the protozoan Babesia bovis. Bioinformatic analysis of the Bartonella henselae genome revealed an FBPA homolog. The B. henselae FBPA enzyme was recombinantly expressed and purified for X-ray crystallographic studies. The purified enzyme crystallized in the apo form but failed to diffract; however, well diffracting crystals could be obtained by cocrystallization in the presence of the native substrate fructose 1,6-bisphosphate. A data set to 2.35 A resolution was collected from a single crystal at 100 K. The crystal belonged to the orthorhombic space group P2(1)2(1)2(1), with unit-cell parameters a = 72.39, b = 127.71, c = 157.63 A. The structure was refined to a final free R factor of 22.2%. The structure shares the typical barrel tertiary structure and tetrameric quaternary structure reported for previous FBPA structures and exhibits the same Schiff base in the active site. Structure of fructose bisphosphate aldolase from Bartonella henselae bound to fructose 1,6-bisphosphate.,Gardberg A, Abendroth J, Bhandari J, Sankaran B, Staker B Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Sep 1;67(Pt, 9):1051-4. Epub 2011 Aug 13. PMID:21904049[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||