2h7f: Difference between revisions
No edit summary |
No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
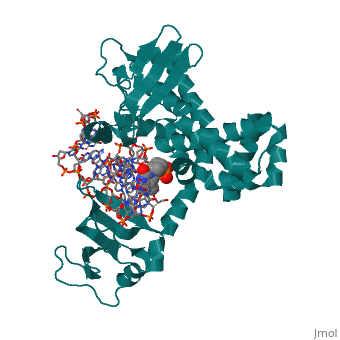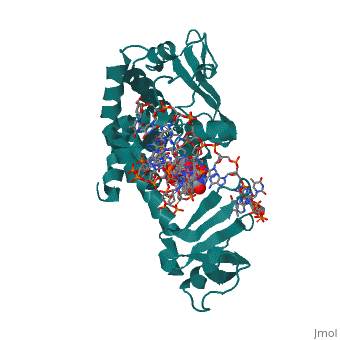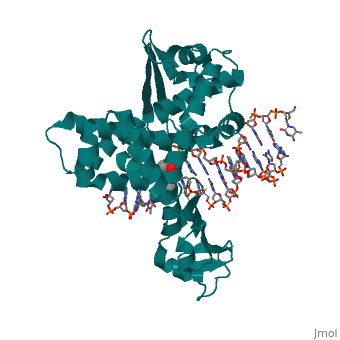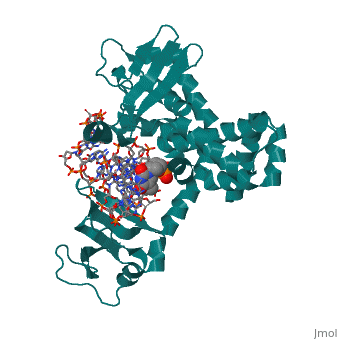
==Structure of variola topoisomerase covalently bound to DNA== | |||
<StructureSection load='2h7f' size='340' side='right'caption='[[2h7f]], [[Resolution|resolution]] 2.70Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[2h7f]] is a 3 chain structure with sequence from [https://en.wikipedia.org/wiki/Variola_virus Variola virus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2H7F OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2H7F FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.7Å</td></tr> | |||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=PTR:O-PHOSPHOTYROSINE'>PTR</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2h7f FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2h7f OCA], [https://pdbe.org/2h7f PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2h7f RCSB], [https://www.ebi.ac.uk/pdbsum/2h7f PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2h7f ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/TOP1_VAR67 TOP1_VAR67] Releases the supercoiling and torsional tension of DNA introduced during the DNA replication and transcription by transiently cleaving and rejoining one strand of the DNA duplex. Introduces a single-strand break via transesterification at the specific target site 5'-[CT]CCTTp site in duplex DNA. The scissile phosphodiester is attacked by the catalytic tyrosine of the enzyme, resulting in the formation of a DNA-(3'-phosphotyrosyl)-enzyme intermediate and the expulsion of a 5'-OH DNA strand. The free DNA strand then undergoes passage around the unbroken strand thus removing DNA supercoils. Finally, in the religation step, the DNA 5'-OH attacks the covalent intermediate to expel the active-site tyrosine and restore the DNA phosphodiester backbone (By similarity). | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/h7/2h7f_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview03.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2h7f ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Although smallpox has been eradicated from the human population, it is presently feared as a possible agent of bioterrorism. The smallpox virus codes for its own topoisomerase enzyme that differs from its cellular counterpart by requiring a specific DNA sequence for activation of catalysis. Here we present crystal structures of the smallpox virus topoisomerase enzyme bound both covalently and noncovalently to a specific DNA sequence. These structures reveal the basis for site-specific DNA recognition, and they explain how catalysis is likely activated by formation of a specific enzyme-DNA interface. Unexpectedly, the poxvirus enzyme uses a major groove binding alpha helix that is not present in the human enzyme to recognize part of the core recognition sequence and activate the enzyme for catalysis. The topoisomerase-DNA complex structures also provide a three-dimensional framework that may facilitate the rational design of therapeutic agents to treat poxvirus infections. | |||
Structural basis for specificity in the poxvirus topoisomerase.,Perry K, Hwang Y, Bushman FD, Van Duyne GD Mol Cell. 2006 Aug 4;23(3):343-54. PMID:16885024<ref>PMID:16885024</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 2h7f" style="background-color:#fffaf0;"></div> | |||
==See Also== | ==See Also== | ||
*[[Topoisomerase|Topoisomerase]] | *[[Topoisomerase|Topoisomerase]] | ||
*[[Topoisomerase 3D structures|Topoisomerase 3D structures]] | |||
== | == References == | ||
< | <references/> | ||
[[Category: | __TOC__ | ||
</StructureSection> | |||
[[Category: Large Structures]] | |||
[[Category: Variola virus]] | [[Category: Variola virus]] | ||
[[Category: Bushman | [[Category: Bushman FD]] | ||
[[Category: Hwang Y]] | |||
[[Category: Hwang | [[Category: Perry K]] | ||
[[Category: Perry | [[Category: Van Duyne GD]] | ||
[[Category: | |||
Latest revision as of 04:00, 21 November 2024
Structure of variola topoisomerase covalently bound to DNAStructure of variola topoisomerase covalently bound to DNA
Structural highlights
FunctionTOP1_VAR67 Releases the supercoiling and torsional tension of DNA introduced during the DNA replication and transcription by transiently cleaving and rejoining one strand of the DNA duplex. Introduces a single-strand break via transesterification at the specific target site 5'-[CT]CCTTp site in duplex DNA. The scissile phosphodiester is attacked by the catalytic tyrosine of the enzyme, resulting in the formation of a DNA-(3'-phosphotyrosyl)-enzyme intermediate and the expulsion of a 5'-OH DNA strand. The free DNA strand then undergoes passage around the unbroken strand thus removing DNA supercoils. Finally, in the religation step, the DNA 5'-OH attacks the covalent intermediate to expel the active-site tyrosine and restore the DNA phosphodiester backbone (By similarity). Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedAlthough smallpox has been eradicated from the human population, it is presently feared as a possible agent of bioterrorism. The smallpox virus codes for its own topoisomerase enzyme that differs from its cellular counterpart by requiring a specific DNA sequence for activation of catalysis. Here we present crystal structures of the smallpox virus topoisomerase enzyme bound both covalently and noncovalently to a specific DNA sequence. These structures reveal the basis for site-specific DNA recognition, and they explain how catalysis is likely activated by formation of a specific enzyme-DNA interface. Unexpectedly, the poxvirus enzyme uses a major groove binding alpha helix that is not present in the human enzyme to recognize part of the core recognition sequence and activate the enzyme for catalysis. The topoisomerase-DNA complex structures also provide a three-dimensional framework that may facilitate the rational design of therapeutic agents to treat poxvirus infections. Structural basis for specificity in the poxvirus topoisomerase.,Perry K, Hwang Y, Bushman FD, Van Duyne GD Mol Cell. 2006 Aug 4;23(3):343-54. PMID:16885024[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||