2exs: Difference between revisions
No edit summary |
No edit summary |
||
| (10 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==TRAP3 (engineered TRAP)== | |||
<StructureSection load='2exs' size='340' side='right'caption='[[2exs]], [[Resolution|resolution]] 2.00Å' scene=''> | |||
== Structural highlights == | |||
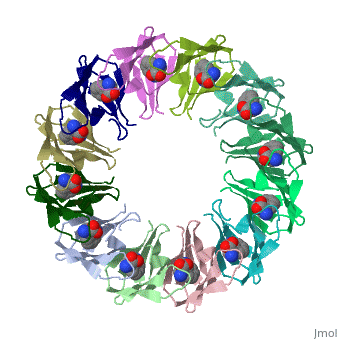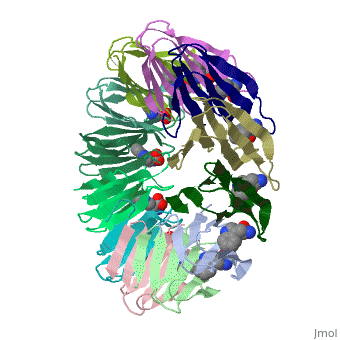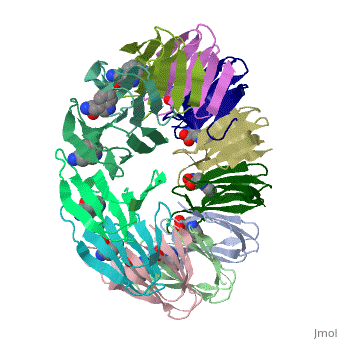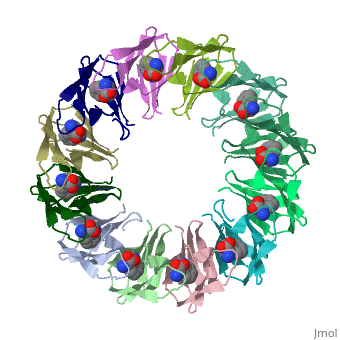
<table><tr><td colspan='2'>[[2exs]] is a 3 chain structure with sequence from [https://en.wikipedia.org/wiki/Geobacillus_stearothermophilus Geobacillus stearothermophilus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2EXS OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2EXS FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2Å</td></tr> | |||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=MSE:SELENOMETHIONINE'>MSE</scene>, <scene name='pdbligand=TRP:TRYPTOPHAN'>TRP</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2exs FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2exs OCA], [https://pdbe.org/2exs PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2exs RCSB], [https://www.ebi.ac.uk/pdbsum/2exs PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2exs ProSAT]</span></td></tr> | |||
</table> | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/ex/2exs_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview03.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2exs ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
The protein TRAP (trp RNA binding attenuation protein) forms a highly thermostable ring-shaped 11-mer. By linking in tandem two, three, or four DNA sequences encoding TRAP monomers, we have engineered new rings that consist of 12 TRAP subunits and bind 12 ligand molecules. The hydrogen bonding pattern and buried surface area within and between subunits are essentially identical between the 11-mer and 12-mer crystal structures. Why do the artificial proteins choose to make single 12-mer rings? The 12-mer rings are highly sterically strained by their peptide linkers and far from thermostable. That proteins choose to adopt a strained conformation of few subunits rather than an unstrained one with 11 subunits demonstrates the importance of entropic factors in controlling protein-protein interactions in general. | |||
Rounding up: Engineering 12-membered rings from the cyclic 11-mer TRAP.,Heddle JG, Yokoyama T, Yamashita I, Park SY, Tame JR Structure. 2006 May;14(5):925-33. PMID:16698553<ref>PMID:16698553</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 2exs" style="background-color:#fffaf0;"></div> | |||
== | ==See Also== | ||
*[[Tryptophan RNA-binding attenuation protein|Tryptophan RNA-binding attenuation protein]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
[[Category: Geobacillus stearothermophilus]] | [[Category: Geobacillus stearothermophilus]] | ||
[[Category: | [[Category: Large Structures]] | ||
[[Category: Heddle | [[Category: Heddle JG]] | ||
[[Category: Park | [[Category: Park SY]] | ||
[[Category: Tame | [[Category: Tame JRH]] | ||
[[Category: Yamashita | [[Category: Yamashita I]] | ||
[[Category: Yokoyama | [[Category: Yokoyama T]] | ||
Latest revision as of 03:54, 21 November 2024
TRAP3 (engineered TRAP)TRAP3 (engineered TRAP)
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe protein TRAP (trp RNA binding attenuation protein) forms a highly thermostable ring-shaped 11-mer. By linking in tandem two, three, or four DNA sequences encoding TRAP monomers, we have engineered new rings that consist of 12 TRAP subunits and bind 12 ligand molecules. The hydrogen bonding pattern and buried surface area within and between subunits are essentially identical between the 11-mer and 12-mer crystal structures. Why do the artificial proteins choose to make single 12-mer rings? The 12-mer rings are highly sterically strained by their peptide linkers and far from thermostable. That proteins choose to adopt a strained conformation of few subunits rather than an unstrained one with 11 subunits demonstrates the importance of entropic factors in controlling protein-protein interactions in general. Rounding up: Engineering 12-membered rings from the cyclic 11-mer TRAP.,Heddle JG, Yokoyama T, Yamashita I, Park SY, Tame JR Structure. 2006 May;14(5):925-33. PMID:16698553[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||