1czf: Difference between revisions
No edit summary |
No edit summary |
||
| (11 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
< | ==ENDO-POLYGALACTURONASE II FROM ASPERGILLUS NIGER== | ||
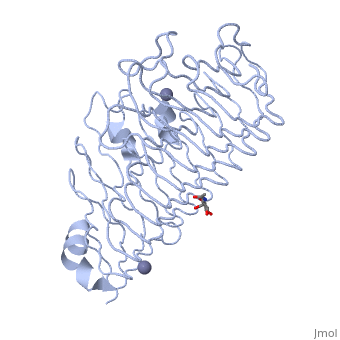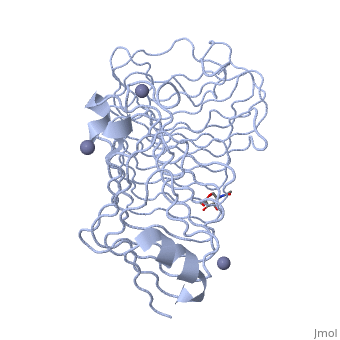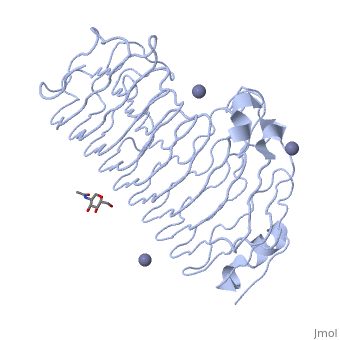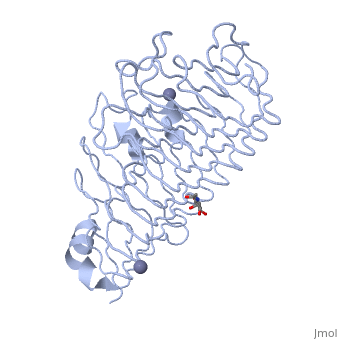
<StructureSection load='1czf' size='340' side='right'caption='[[1czf]], [[Resolution|resolution]] 1.68Å' scene=''> | |||
You may | == Structural highlights == | ||
or the | <table><tr><td colspan='2'>[[1czf]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Aspergillus_niger Aspergillus niger]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1CZF OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1CZF FirstGlance]. <br> | ||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.68Å</td></tr> | |||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene>, <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1czf FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1czf OCA], [https://pdbe.org/1czf PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1czf RCSB], [https://www.ebi.ac.uk/pdbsum/1czf PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1czf ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/PGLR2_ASPNA PGLR2_ASPNA] Involved in maceration and soft-rotting of plant tissue. Hydrolyzes the 1,4-alpha glycosidic bonds of de-esterified pectate in the smooth region of the plant cell wall. | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/cz/1czf_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview03.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1czf ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Polygalacturonases specifically hydrolyze polygalacturonate, a major constituent of plant cell wall pectin. To understand the catalytic mechanism and substrate and product specificity of these enzymes, we have solved the x-ray structure of endopolygalacturonase II of Aspergillus niger and we have carried out site-directed mutagenesis studies. The enzyme folds into a right-handed parallel beta-helix with 10 complete turns. The beta-helix is composed of four parallel beta-sheets, and has one very small alpha-helix near the N terminus, which shields the enzyme's hydrophobic core. Loop regions form a cleft on the exterior of the beta-helix. Site-directed mutagenesis of Asp(180), Asp(201), Asp(202), His(223), Arg(256), and Lys(258), which are located in this cleft, results in a severe reduction of activity, demonstrating that these residues are important for substrate binding and/or catalysis. The juxtaposition of the catalytic residues differs from that normally encountered in inverting glycosyl hydrolases. A comparison of the endopolygalacturonase II active site with that of the P22 tailspike rhamnosidase suggests that Asp(180) and Asp(202) activate the attacking nucleophilic water molecule, while Asp(201) protonates the glycosidic oxygen of the scissile bond. | |||
1.68-A crystal structure of endopolygalacturonase II from Aspergillus niger and identification of active site residues by site-directed mutagenesis.,van Santen Y, Benen JA, Schroter KH, Kalk KH, Armand S, Visser J, Dijkstra BW J Biol Chem. 1999 Oct 22;274(43):30474-80. PMID:10521427<ref>PMID:10521427</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 1czf" style="background-color:#fffaf0;"></div> | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
== | |||
== | |||
[[Category: Aspergillus niger]] | [[Category: Aspergillus niger]] | ||
[[Category: | [[Category: Large Structures]] | ||
[[Category: Dijkstra BW]] | |||
[[Category: Dijkstra | [[Category: Kalk KH]] | ||
[[Category: Kalk | [[Category: Van Santen Y]] | ||
[[Category: Santen | |||
Latest revision as of 09:31, 30 October 2024
ENDO-POLYGALACTURONASE II FROM ASPERGILLUS NIGERENDO-POLYGALACTURONASE II FROM ASPERGILLUS NIGER
Structural highlights
FunctionPGLR2_ASPNA Involved in maceration and soft-rotting of plant tissue. Hydrolyzes the 1,4-alpha glycosidic bonds of de-esterified pectate in the smooth region of the plant cell wall. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedPolygalacturonases specifically hydrolyze polygalacturonate, a major constituent of plant cell wall pectin. To understand the catalytic mechanism and substrate and product specificity of these enzymes, we have solved the x-ray structure of endopolygalacturonase II of Aspergillus niger and we have carried out site-directed mutagenesis studies. The enzyme folds into a right-handed parallel beta-helix with 10 complete turns. The beta-helix is composed of four parallel beta-sheets, and has one very small alpha-helix near the N terminus, which shields the enzyme's hydrophobic core. Loop regions form a cleft on the exterior of the beta-helix. Site-directed mutagenesis of Asp(180), Asp(201), Asp(202), His(223), Arg(256), and Lys(258), which are located in this cleft, results in a severe reduction of activity, demonstrating that these residues are important for substrate binding and/or catalysis. The juxtaposition of the catalytic residues differs from that normally encountered in inverting glycosyl hydrolases. A comparison of the endopolygalacturonase II active site with that of the P22 tailspike rhamnosidase suggests that Asp(180) and Asp(202) activate the attacking nucleophilic water molecule, while Asp(201) protonates the glycosidic oxygen of the scissile bond. 1.68-A crystal structure of endopolygalacturonase II from Aspergillus niger and identification of active site residues by site-directed mutagenesis.,van Santen Y, Benen JA, Schroter KH, Kalk KH, Armand S, Visser J, Dijkstra BW J Biol Chem. 1999 Oct 22;274(43):30474-80. PMID:10521427[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. References |
| ||||||||||||||||||