2ju1: Difference between revisions
No edit summary |
No edit summary |
||
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
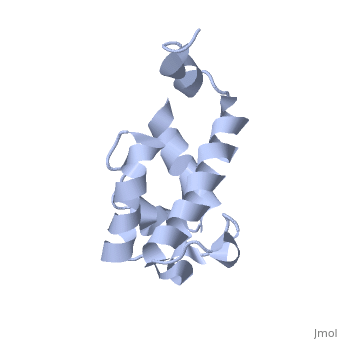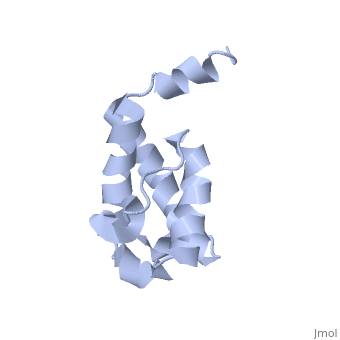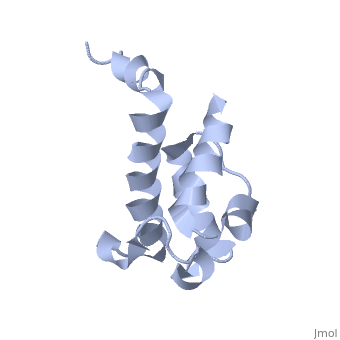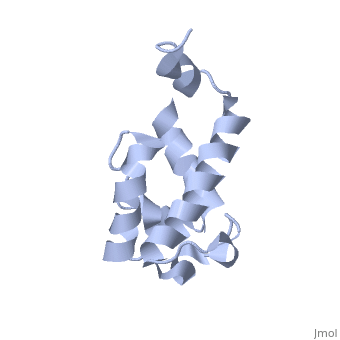
==Solution structure of acyl carrier protein domain from module 2 of 6-deoxyerythronolide B synthase (DEBS)== | |||
<StructureSection load='2ju1' size='340' side='right'caption='[[2ju1]]' scene=''> | |||
| | == Structural highlights == | ||
<table><tr><td colspan='2'>[[2ju1]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Saccharopolyspora_erythraea Saccharopolyspora erythraea]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2JU1 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2JU1 FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">Solution NMR</td></tr> | |||
| | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2ju1 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2ju1 OCA], [https://pdbe.org/2ju1 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2ju1 RCSB], [https://www.ebi.ac.uk/pdbsum/2ju1 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2ju1 ProSAT]</span></td></tr> | ||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/ERYA1_SACER ERYA1_SACER] | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/ju/2ju1_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2ju1 ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Polyketides are a medicinally important class of natural products. The architecture of modular polyketide synthases (PKSs), composed of multiple covalently linked domains grouped into modules, provides an attractive framework for engineering novel polyketide-producing assemblies. However, impaired domain-domain interactions can compromise the efficiency of engineered polyketide biosynthesis. To facilitate the study of these domain-domain interactions, we have used nuclear magnetic resonance (NMR) spectroscopy to determine the first solution structure of an acyl carrier protein (ACP) domain from a modular PKS, 6-deoxyerythronolide B synthase (DEBS). The tertiary fold of this 10-kD domain is a three-helical bundle; an additional short helix in the second loop also contributes to the core helical packing. Superposition of residues 14-94 of the ensemble on the mean structure yields an average atomic RMSD of 0.64 +/- 0.09 Angstrom for the backbone atoms (1.21 +/- 0.13 Angstrom for all non-hydrogen atoms). The three major helices superimpose with a backbone RMSD of 0.48 +/- 0.10 Angstrom (0.99 +/- 0.11 Angstrom for non-hydrogen atoms). Based on this solution structure, homology models were constructed for five other DEBS ACP domains. Comparison of their steric and electrostatic surfaces at the putative interaction interface (centered on helix II) suggests a model for protein-protein recognition of ACP domains, consistent with the previously observed specificity. Site-directed mutagenesis experiments indicate that two of the identified residues influence the specificity of ACP recognition. | |||
Solution structure and proposed domain domain recognition interface of an acyl carrier protein domain from a modular polyketide synthase.,Alekseyev VY, Liu CW, Cane DE, Puglisi JD, Khosla C Protein Sci. 2007 Oct;16(10):2093-107. PMID:17893358<ref>PMID:17893358</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 2ju1" style="background-color:#fffaf0;"></div> | |||
== | ==See Also== | ||
*[[6-deoxyerythronolide B synthase (DEBS)|6-deoxyerythronolide B synthase (DEBS)]] | |||
*[[6-deoxyerythronolide B synthase 3D structures|6-deoxyerythronolide B synthase 3D structures]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
== | </StructureSection> | ||
[[Category: Large Structures]] | |||
[[Category: | |||
[[Category: Saccharopolyspora erythraea]] | [[Category: Saccharopolyspora erythraea]] | ||
[[Category: Alekseyev VY]] | |||
[[Category: Alekseyev | [[Category: Khosla C]] | ||
[[Category: Khosla | [[Category: Liu CW]] | ||
[[Category: Liu | [[Category: Puglisi JD]] | ||
[[Category: Puglisi | |||
Latest revision as of 22:05, 29 May 2024
Solution structure of acyl carrier protein domain from module 2 of 6-deoxyerythronolide B synthase (DEBS)Solution structure of acyl carrier protein domain from module 2 of 6-deoxyerythronolide B synthase (DEBS)
Structural highlights
FunctionEvolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedPolyketides are a medicinally important class of natural products. The architecture of modular polyketide synthases (PKSs), composed of multiple covalently linked domains grouped into modules, provides an attractive framework for engineering novel polyketide-producing assemblies. However, impaired domain-domain interactions can compromise the efficiency of engineered polyketide biosynthesis. To facilitate the study of these domain-domain interactions, we have used nuclear magnetic resonance (NMR) spectroscopy to determine the first solution structure of an acyl carrier protein (ACP) domain from a modular PKS, 6-deoxyerythronolide B synthase (DEBS). The tertiary fold of this 10-kD domain is a three-helical bundle; an additional short helix in the second loop also contributes to the core helical packing. Superposition of residues 14-94 of the ensemble on the mean structure yields an average atomic RMSD of 0.64 +/- 0.09 Angstrom for the backbone atoms (1.21 +/- 0.13 Angstrom for all non-hydrogen atoms). The three major helices superimpose with a backbone RMSD of 0.48 +/- 0.10 Angstrom (0.99 +/- 0.11 Angstrom for non-hydrogen atoms). Based on this solution structure, homology models were constructed for five other DEBS ACP domains. Comparison of their steric and electrostatic surfaces at the putative interaction interface (centered on helix II) suggests a model for protein-protein recognition of ACP domains, consistent with the previously observed specificity. Site-directed mutagenesis experiments indicate that two of the identified residues influence the specificity of ACP recognition. Solution structure and proposed domain domain recognition interface of an acyl carrier protein domain from a modular polyketide synthase.,Alekseyev VY, Liu CW, Cane DE, Puglisi JD, Khosla C Protein Sci. 2007 Oct;16(10):2093-107. PMID:17893358[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||