1m4p: Difference between revisions
No edit summary |
No edit summary |
||
| (11 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
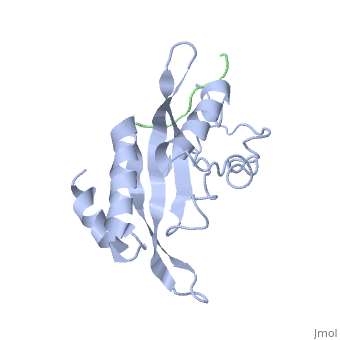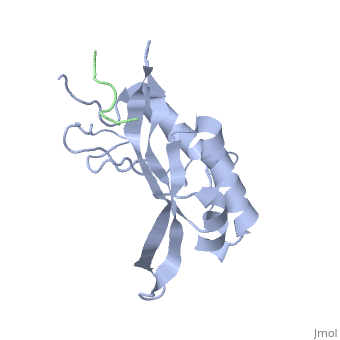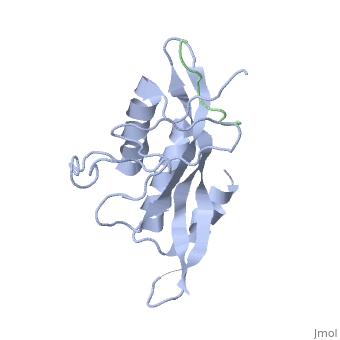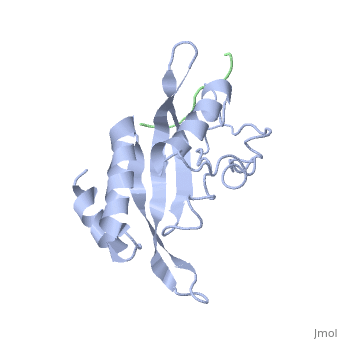
< | ==Structure of the Tsg101 UEV domain in complex with a HIV-1 PTAP "late domain" peptide, DYANA Ensemble== | ||
<StructureSection load='1m4p' size='340' side='right'caption='[[1m4p]]' scene=''> | |||
You may | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1m4p]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] and [https://en.wikipedia.org/wiki/Human_immunodeficiency_virus_1 Human immunodeficiency virus 1]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1M4P OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1M4P FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">Solution NMR</td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1m4p FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1m4p OCA], [https://pdbe.org/1m4p PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1m4p RCSB], [https://www.ebi.ac.uk/pdbsum/1m4p PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1m4p ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/Q9IF21_9HIV1 Q9IF21_9HIV1] | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/m4/1m4p_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1m4p ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
The structural proteins of HIV and Ebola display PTAP peptide motifs (termed 'late domains') that recruit the human protein Tsg101 to facilitate virus budding. Here we present the solution structure of the UEV (ubiquitin E2 variant) binding domain of Tsg101 in complex with a PTAP peptide that spans the late domain of HIV-1 p6(Gag). The UEV domain of Tsg101 resembles E2 ubiquitin-conjugating enzymes, and the PTAP peptide binds in a bifurcated groove above the vestigial enzyme active site. Each PTAP residue makes important contacts, and the Ala 9-Pro 10 dipeptide binds in a deep pocket of the UEV domain that resembles the X-Pro binding pockets of SH3 and WW domains. The structure reveals the molecular basis of HIV PTAP late domain function and represents an attractive starting point for the design of novel inhibitors of virus budding. | |||
Structure of the Tsg101 UEV domain in complex with the PTAP motif of the HIV-1 p6 protein.,Pornillos O, Alam SL, Davis DR, Sundquist WI Nat Struct Biol. 2002 Nov;9(11):812-7. PMID:12379843<ref>PMID:12379843</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 1m4p" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Tumor susceptibility gene 101|Tumor susceptibility gene 101]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
== | |||
== | |||
< | |||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Human immunodeficiency virus 1]] | [[Category: Human immunodeficiency virus 1]] | ||
[[Category: | [[Category: Large Structures]] | ||
[[Category: | [[Category: Alam SL]] | ||
[[Category: | [[Category: Davis DR]] | ||
[[Category: | [[Category: Pornillos O]] | ||
[[Category: | [[Category: Sundquist WI]] | ||
Latest revision as of 11:48, 22 May 2024
Structure of the Tsg101 UEV domain in complex with a HIV-1 PTAP "late domain" peptide, DYANA EnsembleStructure of the Tsg101 UEV domain in complex with a HIV-1 PTAP "late domain" peptide, DYANA Ensemble
Structural highlights
FunctionEvolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe structural proteins of HIV and Ebola display PTAP peptide motifs (termed 'late domains') that recruit the human protein Tsg101 to facilitate virus budding. Here we present the solution structure of the UEV (ubiquitin E2 variant) binding domain of Tsg101 in complex with a PTAP peptide that spans the late domain of HIV-1 p6(Gag). The UEV domain of Tsg101 resembles E2 ubiquitin-conjugating enzymes, and the PTAP peptide binds in a bifurcated groove above the vestigial enzyme active site. Each PTAP residue makes important contacts, and the Ala 9-Pro 10 dipeptide binds in a deep pocket of the UEV domain that resembles the X-Pro binding pockets of SH3 and WW domains. The structure reveals the molecular basis of HIV PTAP late domain function and represents an attractive starting point for the design of novel inhibitors of virus budding. Structure of the Tsg101 UEV domain in complex with the PTAP motif of the HIV-1 p6 protein.,Pornillos O, Alam SL, Davis DR, Sundquist WI Nat Struct Biol. 2002 Nov;9(11):812-7. PMID:12379843[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||