2lnm: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Solution structure of the C-terminal NP-repeat domain of Tic40, a co-chaperone during protein import into chloroplasts== | ==Solution structure of the C-terminal NP-repeat domain of Tic40, a co-chaperone during protein import into chloroplasts== | ||
<StructureSection load='2lnm' size='340' side='right'caption='[[2lnm | <StructureSection load='2lnm' size='340' side='right'caption='[[2lnm]]' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[2lnm]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/ | <table><tr><td colspan='2'>[[2lnm]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Arabidopsis_thaliana Arabidopsis thaliana]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2LNM OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2LNM FirstGlance]. <br> | ||
</td></tr><tr id=' | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">Solution NMR</td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2lnm FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2lnm OCA], [https://pdbe.org/2lnm PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2lnm RCSB], [https://www.ebi.ac.uk/pdbsum/2lnm PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2lnm ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2lnm FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2lnm OCA], [https://pdbe.org/2lnm PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2lnm RCSB], [https://www.ebi.ac.uk/pdbsum/2lnm PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2lnm ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
[https://www.uniprot.org/uniprot/TIC40_ARATH TIC40_ARATH] Involved in protein precursor import into chloroplasts. Part of the motor complex consisting of a co-chaperone (TIC40) and a chaperone (HSP93) associated with the import channel (TIC110). Causes the release of bound transit peptides from TIC110 and stimulates ATP hydrolysis by HSP93. Involved in reinsertion of proteins from the chloroplast stroma into the inner membrane.<ref>PMID:12805212</ref> <ref>PMID:15829604</ref> <ref>PMID:15659100</ref> <ref>PMID:17158958</ref> <ref>PMID:17535810</ref> <ref>PMID:18657235</ref> | |||
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
== Publication Abstract from PubMed == | == Publication Abstract from PubMed == | ||
| Line 25: | Line 25: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: | [[Category: Arabidopsis thaliana]] | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
[[Category: Chen | [[Category: Chen C]] | ||
[[Category: Kao | [[Category: Kao Y]] | ||
Latest revision as of 08:47, 15 May 2024
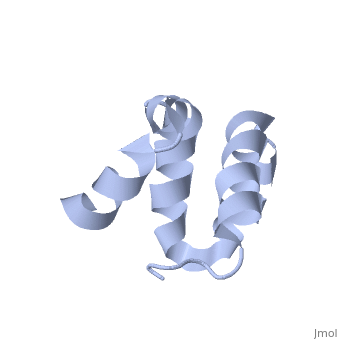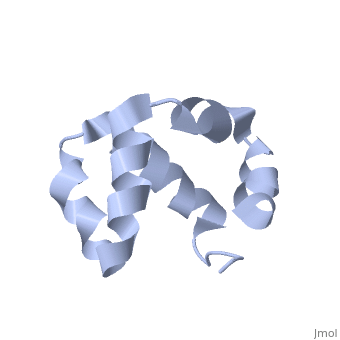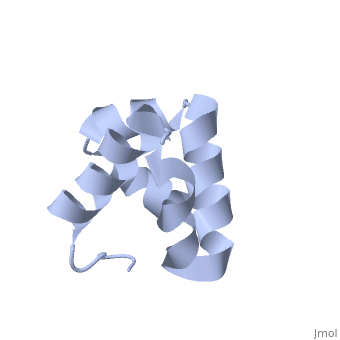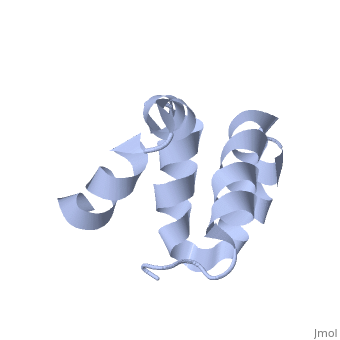
Solution structure of the C-terminal NP-repeat domain of Tic40, a co-chaperone during protein import into chloroplastsSolution structure of the C-terminal NP-repeat domain of Tic40, a co-chaperone during protein import into chloroplasts
Structural highlights
FunctionTIC40_ARATH Involved in protein precursor import into chloroplasts. Part of the motor complex consisting of a co-chaperone (TIC40) and a chaperone (HSP93) associated with the import channel (TIC110). Causes the release of bound transit peptides from TIC110 and stimulates ATP hydrolysis by HSP93. Involved in reinsertion of proteins from the chloroplast stroma into the inner membrane.[1] [2] [3] [4] [5] [6] Publication Abstract from PubMedChloroplasts protein precursors translated in the cytosol traverse the membranes to reach their intended destination with the help of translocon complexes called translocon at the outer envelope of chloroplasts and translocon at the inner envelope of chloroplasts (TIC), respectively. Two components of the TIC translocon, Tic110 and Tic40, which combine with Hsp93 (ClpC), are involved in protein translocation across the inner membrane into the stroma. The C-terminal NP-repeat domain of Tic40 (Tic40-NP) is homologous to the DP-repeat domain of co-chaperones Hsp70-interacting and Hsp70/Hsp90-organizing proteins. Interaction of Tic40-NP and Hsp93 stimulates ATP hydrolysis of Hsp93, but the hydrolysis is abolished in both N320A and N329A mutants of Tic40-NP. Here, we determined the nuclear magnetic resonance structure of Tic40-NP, which mainly consists of five alpha-helices stabilized by two hydrophobic cores. In addition, chemical shift perturbation results suggested that some residues at alpha1 and alpha5, as well as residues Asn320 and Asn329, cause conformational change on the two mutants, which may subsequently affect their binding to Hsp93. We provide valuable information for further investigating how Tic40-NP interacts with Hsp93. Solution structure of the C-terminal NP-repeat domain of Tic40, a co-chaperone during protein import into chloroplasts.,Kao YF, Lou YC, Yeh YH, Hsiao CD, Chen C J Biochem. 2012 Nov;152(5):443-51. doi: 10.1093/jb/mvs086. Epub 2012 Aug 10. PMID:22888115[7] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||