2cns: Difference between revisions
No edit summary |
No edit summary |
||
| (18 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
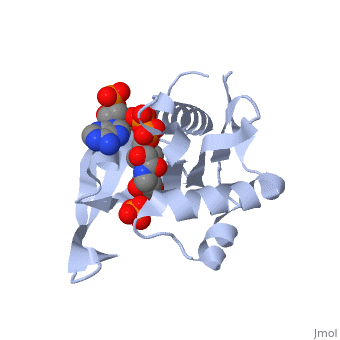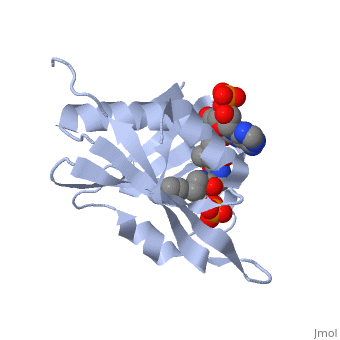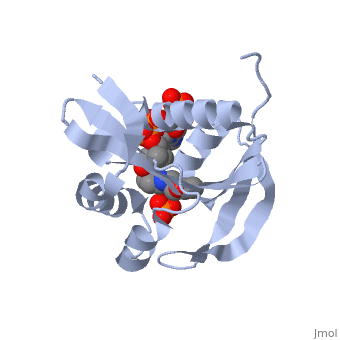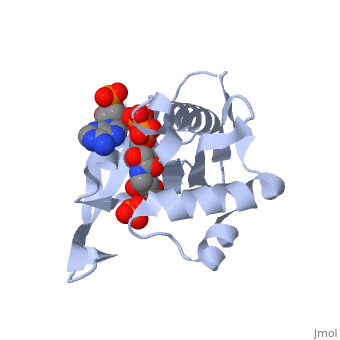
== | ==RimI - Ribosomal S18 N-alpha-protein acetyltransferase in complex with acetylCoA.== | ||
<StructureSection load='2cns' size='340' side='right'caption='[[2cns]], [[Resolution|resolution]] 2.50Å' scene=''> | |||
[ | == Structural highlights == | ||
<table><tr><td colspan='2'>[[2cns]] is a 3 chain structure with sequence from [https://en.wikipedia.org/wiki/Salmonella_enterica_subsp._enterica_serovar_Typhimurium_str._LT2 Salmonella enterica subsp. enterica serovar Typhimurium str. LT2]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2CNS OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2CNS FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.5Å</td></tr> | |||
[[ | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=ACO:ACETYL+COENZYME+*A'>ACO</scene>, <scene name='pdbligand=PO4:PHOSPHATE+ION'>PO4</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2cns FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2cns OCA], [https://pdbe.org/2cns PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2cns RCSB], [https://www.ebi.ac.uk/pdbsum/2cns PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2cns ProSAT]</span></td></tr> | |||
[ | </table> | ||
[[ | == Function == | ||
[ | [https://www.uniprot.org/uniprot/RIMI_SALTY RIMI_SALTY] Acetylates the N-terminal alanine of ribosomal protein S18.[HAMAP-Rule:MF_02210]<ref>PMID:18596200</ref> | ||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/cn/2cns_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2cns ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
The three ribosomal proteins L7, S5, and S18 are included in the rare subset of prokaryotic proteins that are known to be N(alpha)-acetylated. The GCN5-related N-acetyltransferase (GNAT) protein RimI, responsible for the N(alpha)-acetylation of the ribosomal protein S18, was cloned from Salmonella typhimurium LT2 (RimI(ST)), overexpressed, and purified to homogeneity. Steady-state kinetic parameters for RimI(ST) were determined for AcCoA and a peptide substrate consisting of the first six amino acids of the target protein S18. The crystal structure of RimI(ST) was determined in complex with CoA, AcCoA, and a CoA-S-acetyl-ARYFRR bisubstrate inhibitor. The structures are consistent with a direct nucleophilic addition-elimination mechanism with Glu103 and Tyr115 acting as the catalytic base and acid, respectively. The RimI(ST)-bisubstrate complex suggests that several residues change conformation upon interacting with the N terminus of S18, including Glu103, the proposed active site base, facilitating proton exchange and catalysis. | |||
Crystal structure of RimI from Salmonella typhimurium LT2, the GNAT responsible for N(alpha)-acetylation of ribosomal protein S18.,Vetting MW, Bareich DC, Yu M, Blanchard JS Protein Sci. 2008 Oct;17(10):1781-90. Epub 2008 Jul 2. PMID:18596200<ref>PMID:18596200</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 2cns" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Ribosomal protein S18|Ribosomal protein S18]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
[[Category: Large Structures]] | |||
[[Category: Salmonella enterica subsp. enterica serovar Typhimurium str. LT2]] | |||
[[Category: Bareich DC]] | |||
[[Category: Blanchard JS]] | |||
[[Category: Vetting MW]] | |||
[[Category: Yu M]] | |||
Latest revision as of 12:28, 9 May 2024
RimI - Ribosomal S18 N-alpha-protein acetyltransferase in complex with acetylCoA.RimI - Ribosomal S18 N-alpha-protein acetyltransferase in complex with acetylCoA.
Structural highlights
FunctionRIMI_SALTY Acetylates the N-terminal alanine of ribosomal protein S18.[HAMAP-Rule:MF_02210][1] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe three ribosomal proteins L7, S5, and S18 are included in the rare subset of prokaryotic proteins that are known to be N(alpha)-acetylated. The GCN5-related N-acetyltransferase (GNAT) protein RimI, responsible for the N(alpha)-acetylation of the ribosomal protein S18, was cloned from Salmonella typhimurium LT2 (RimI(ST)), overexpressed, and purified to homogeneity. Steady-state kinetic parameters for RimI(ST) were determined for AcCoA and a peptide substrate consisting of the first six amino acids of the target protein S18. The crystal structure of RimI(ST) was determined in complex with CoA, AcCoA, and a CoA-S-acetyl-ARYFRR bisubstrate inhibitor. The structures are consistent with a direct nucleophilic addition-elimination mechanism with Glu103 and Tyr115 acting as the catalytic base and acid, respectively. The RimI(ST)-bisubstrate complex suggests that several residues change conformation upon interacting with the N terminus of S18, including Glu103, the proposed active site base, facilitating proton exchange and catalysis. Crystal structure of RimI from Salmonella typhimurium LT2, the GNAT responsible for N(alpha)-acetylation of ribosomal protein S18.,Vetting MW, Bareich DC, Yu M, Blanchard JS Protein Sci. 2008 Oct;17(10):1781-90. Epub 2008 Jul 2. PMID:18596200[2] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||