2ysu: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
<StructureSection load='2ysu' size='340' side='right'caption='[[2ysu]], [[Resolution|resolution]] 3.50Å' scene=''> | <StructureSection load='2ysu' size='340' side='right'caption='[[2ysu]], [[Resolution|resolution]] 3.50Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
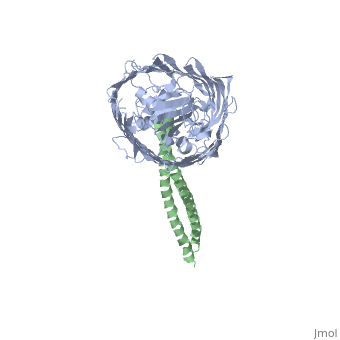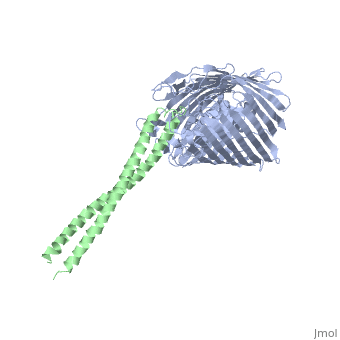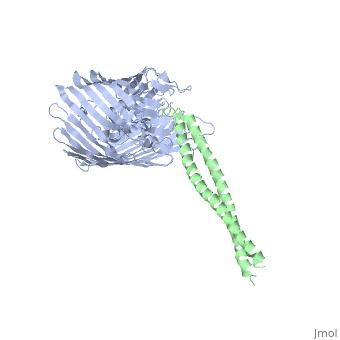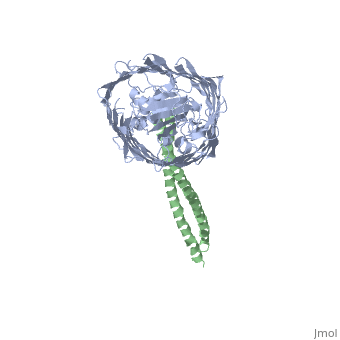
<table><tr><td colspan='2'>[[2ysu]] is a 2 chain structure with sequence from [ | <table><tr><td colspan='2'>[[2ysu]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2YSU OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2YSU FirstGlance]. <br> | ||
</td></tr><tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.5Å</td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2ysu FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2ysu OCA], [https://pdbe.org/2ysu PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2ysu RCSB], [https://www.ebi.ac.uk/pdbsum/2ysu PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2ysu ProSAT]</span></td></tr> | |||
</table> | </table> | ||
== Function == | == Function == | ||
[ | [https://www.uniprot.org/uniprot/BTUB_ECOLI BTUB_ECOLI] Involved in the active translocation of vitamin B12 (cyanocobalamin) across the outer membrane to the periplasmic space. It derives its energy for transport by interacting with the trans-periplasmic membrane protein TonB. Is also a receptor for bacteriophages BF23 and C1, and for A and E colicins.[HAMAP-Rule:MF_01531] | ||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 18: | Line 19: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2ysu ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2ysu ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
==See Also== | ==See Also== | ||
*[[BtuB 3D structures|BtuB 3D structures]] | *[[BtuB 3D structures|BtuB 3D structures]] | ||
*[[Colicin 3D structures|Colicin 3D structures]] | *[[Colicin 3D structures|Colicin 3D structures]] | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: | [[Category: Escherichia coli]] | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
[[Category: Cramer | [[Category: Cramer WA]] | ||
[[Category: Sharma | [[Category: Sharma O]] | ||
[[Category: Yamashita | [[Category: Yamashita E]] | ||
Latest revision as of 16:54, 13 March 2024
Structure of the complex between BtuB and Colicin E2 receptor binding domainStructure of the complex between BtuB and Colicin E2 receptor binding domain
Structural highlights
FunctionBTUB_ECOLI Involved in the active translocation of vitamin B12 (cyanocobalamin) across the outer membrane to the periplasmic space. It derives its energy for transport by interacting with the trans-periplasmic membrane protein TonB. Is also a receptor for bacteriophages BF23 and C1, and for A and E colicins.[HAMAP-Rule:MF_01531] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. See Also |
| ||||||||||||||||