4urt: Difference between revisions
New page: '''Unreleased structure''' The entry 4urt is ON HOLD Authors: Finci, L.I., Krueger, N., Sun, X., Zhang, J., Chegkazi, M., Wu, Y., Schenk, G., Mertens, H.D.T., Svergun, D.I., Zhang, Y., ... |
No edit summary |
||
| (10 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
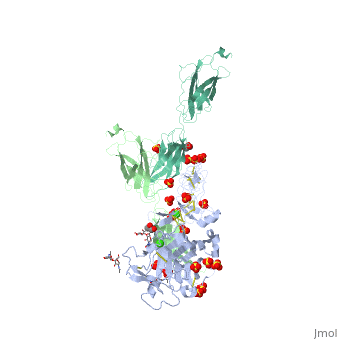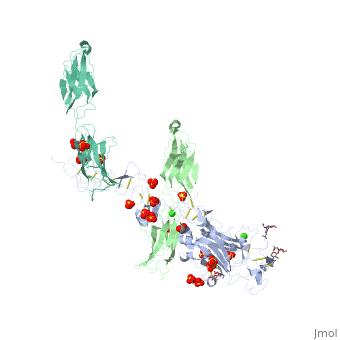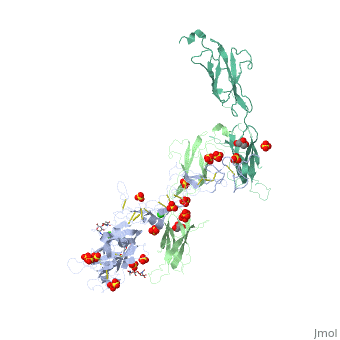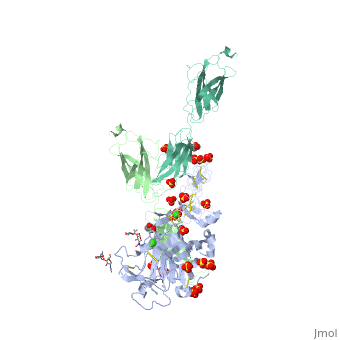
The | ==The crystal structure of a fragment of netrin-1 in complex with FN5- FN6 of DCC== | ||
<StructureSection load='4urt' size='340' side='right'caption='[[4urt]], [[Resolution|resolution]] 3.10Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[4urt]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4URT OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=4URT FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.1Å</td></tr> | |||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=CA:CALCIUM+ION'>CA</scene>, <scene name='pdbligand=CL:CHLORIDE+ION'>CL</scene>, <scene name='pdbligand=GOL:GLYCEROL'>GOL</scene>, <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=4urt FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4urt OCA], [https://pdbe.org/4urt PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=4urt RCSB], [https://www.ebi.ac.uk/pdbsum/4urt PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=4urt ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/NET1_HUMAN NET1_HUMAN] Netrins control guidance of CNS commissural axons and peripheral motor axons. Its association with either DCC or some UNC5 receptors will lead to axon attraction or repulsion, respectively. It also serve as a survival factor via its association with its receptors which prevent the initiation of apoptosis. Involved in tumorigenesis by regulating apoptosis.<ref>PMID:15343335</ref> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Netrin-1 is a guidance cue that can trigger either attraction or repulsion effects on migrating axons of neurons, depending on the repertoire of receptors available on the growth cone. How a single chemotropic molecule can act in such contradictory ways has long been a puzzle at the molecular level. Here we present the crystal structure of netrin-1 in complex with the Deleted in Colorectal Cancer (DCC) receptor. We show that one netrin-1 molecule can simultaneously bind to two DCC molecules through a DCC-specific site and through a unique generic receptor binding site, where sulfate ions staple together positively charged patches on both DCC and netrin-1. Furthermore, we demonstrate that UNC5A can replace DCC on the generic receptor binding site to switch the response from attraction to repulsion. We propose that the modularity of binding allows for the association of other netrin receptors at the generic binding site, eliciting alternative turning responses. | |||
The Crystal Structure of Netrin-1 in Complex with DCC Reveals the Bifunctionality of Netrin-1 As a Guidance Cue.,Finci LI, Kruger N, Sun X, Zhang J, Chegkazi M, Wu Y, Schenk G, Mertens HD, Svergun DI, Zhang Y, Wang JH, Meijers R Neuron. 2014 Aug 20;83(4):839-49. doi: 10.1016/j.neuron.2014.07.010. Epub 2014, Aug 7. PMID:25123307<ref>PMID:25123307</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 4urt" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Netrin|Netrin]] | |||
*[[Netrin receptor|Netrin receptor]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
[[Category: Homo sapiens]] | |||
[[Category: Large Structures]] | |||
[[Category: Chegkazi M]] | |||
[[Category: Finci LI]] | |||
[[Category: Krueger N]] | |||
[[Category: Meijers R]] | |||
[[Category: Mertens HDT]] | |||
[[Category: Schenk G]] | |||
[[Category: Sun X]] | |||
[[Category: Svergun DI]] | |||
[[Category: Wang J-h]] | |||
[[Category: Wu Y]] | |||
[[Category: Zhang J]] | |||
[[Category: Zhang Y]] | |||
Latest revision as of 13:34, 10 January 2024
The crystal structure of a fragment of netrin-1 in complex with FN5- FN6 of DCCThe crystal structure of a fragment of netrin-1 in complex with FN5- FN6 of DCC
Structural highlights
FunctionNET1_HUMAN Netrins control guidance of CNS commissural axons and peripheral motor axons. Its association with either DCC or some UNC5 receptors will lead to axon attraction or repulsion, respectively. It also serve as a survival factor via its association with its receptors which prevent the initiation of apoptosis. Involved in tumorigenesis by regulating apoptosis.[1] Publication Abstract from PubMedNetrin-1 is a guidance cue that can trigger either attraction or repulsion effects on migrating axons of neurons, depending on the repertoire of receptors available on the growth cone. How a single chemotropic molecule can act in such contradictory ways has long been a puzzle at the molecular level. Here we present the crystal structure of netrin-1 in complex with the Deleted in Colorectal Cancer (DCC) receptor. We show that one netrin-1 molecule can simultaneously bind to two DCC molecules through a DCC-specific site and through a unique generic receptor binding site, where sulfate ions staple together positively charged patches on both DCC and netrin-1. Furthermore, we demonstrate that UNC5A can replace DCC on the generic receptor binding site to switch the response from attraction to repulsion. We propose that the modularity of binding allows for the association of other netrin receptors at the generic binding site, eliciting alternative turning responses. The Crystal Structure of Netrin-1 in Complex with DCC Reveals the Bifunctionality of Netrin-1 As a Guidance Cue.,Finci LI, Kruger N, Sun X, Zhang J, Chegkazi M, Wu Y, Schenk G, Mertens HD, Svergun DI, Zhang Y, Wang JH, Meijers R Neuron. 2014 Aug 20;83(4):839-49. doi: 10.1016/j.neuron.2014.07.010. Epub 2014, Aug 7. PMID:25123307[2] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||