3few: Difference between revisions
No edit summary |
No edit summary |
||
| (9 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
< | ==Structure and Function of Colicin S4, a colicin with a duplicated receptor binding domain== | ||
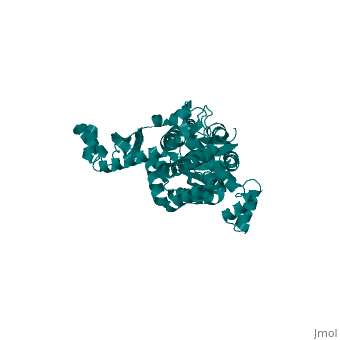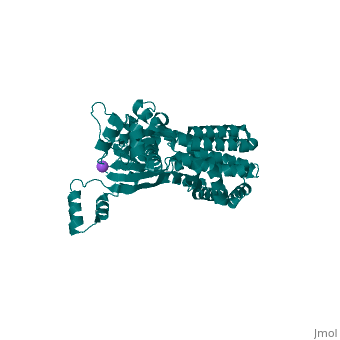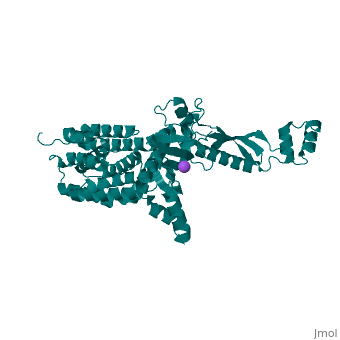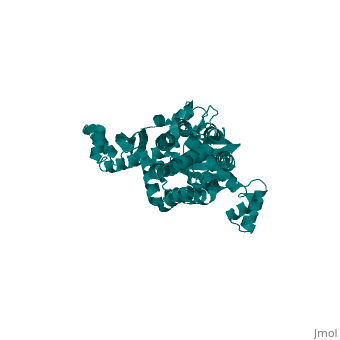
<StructureSection load='3few' size='340' side='right'caption='[[3few]], [[Resolution|resolution]] 2.45Å' scene=''> | |||
You may | == Structural highlights == | ||
or the | <table><tr><td colspan='2'>[[3few]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3FEW OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3FEW FirstGlance]. <br> | ||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.45Å</td></tr> | |||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=NA:SODIUM+ION'>NA</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3few FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3few OCA], [https://pdbe.org/3few PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3few RCSB], [https://www.ebi.ac.uk/pdbsum/3few PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3few ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/Q9XB47_ECOLX Q9XB47_ECOLX] | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/fe/3few_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=3few ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Colicins are plasmid-encoded toxic proteins produced by Escherichia coli strains to kill other E. coli strains that lack the corresponding immunity protein. Colicins intrude into the host cell by exploiting existing transport, diffusion, or efflux systems. We have traced the way colicin S4 takes to execute its function and show that it interacts specifically with OmpW, OmpF, and the Tol system before it inserts its pore-forming domain into the cytoplasmic membrane. The common structural architecture of colicins comprises a translocation, a receptor-binding, and an activity domain. We have solved the crystal structure of colicin S4 to a resolution of 2.5 A, which shows a remarkably compact domain arrangement of four independent domains, including a unique domain duplication of the receptor-binding domain. Finally, we have determined the residues responsible for binding to the receptor OmpW by mutating exposed charged residues in one or both receptor-binding domains. | |||
Structure and function of colicin S4, a colicin with a duplicated receptor-binding domain.,Arnold T, Zeth K, Linke D J Biol Chem. 2009 Mar 6;284(10):6403-13. Epub 2008 Dec 4. PMID:19056731<ref>PMID:19056731</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 3few" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Colicin 3D structures|Colicin 3D structures]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
== | |||
== | |||
< | |||
[[Category: Escherichia coli]] | [[Category: Escherichia coli]] | ||
[[Category: | [[Category: Large Structures]] | ||
[[Category: | [[Category: Arnold T]] | ||
[[Category: | [[Category: Linke D]] | ||
[[Category: | [[Category: Zeth K]] | ||
Latest revision as of 03:31, 28 December 2023
Structure and Function of Colicin S4, a colicin with a duplicated receptor binding domainStructure and Function of Colicin S4, a colicin with a duplicated receptor binding domain
Structural highlights
FunctionEvolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedColicins are plasmid-encoded toxic proteins produced by Escherichia coli strains to kill other E. coli strains that lack the corresponding immunity protein. Colicins intrude into the host cell by exploiting existing transport, diffusion, or efflux systems. We have traced the way colicin S4 takes to execute its function and show that it interacts specifically with OmpW, OmpF, and the Tol system before it inserts its pore-forming domain into the cytoplasmic membrane. The common structural architecture of colicins comprises a translocation, a receptor-binding, and an activity domain. We have solved the crystal structure of colicin S4 to a resolution of 2.5 A, which shows a remarkably compact domain arrangement of four independent domains, including a unique domain duplication of the receptor-binding domain. Finally, we have determined the residues responsible for binding to the receptor OmpW by mutating exposed charged residues in one or both receptor-binding domains. Structure and function of colicin S4, a colicin with a duplicated receptor-binding domain.,Arnold T, Zeth K, Linke D J Biol Chem. 2009 Mar 6;284(10):6403-13. Epub 2008 Dec 4. PMID:19056731[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||