1b3q: Difference between revisions
No edit summary |
No edit summary |
||
| (8 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== | ==CRYSTAL STRUCTURE OF CHEA-289, A SIGNAL TRANSDUCING HISTIDINE KINASE== | ||
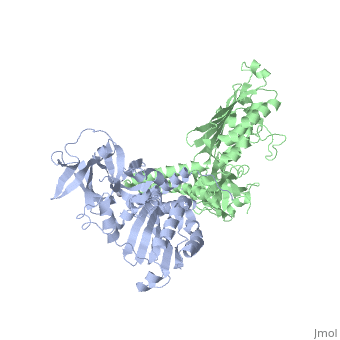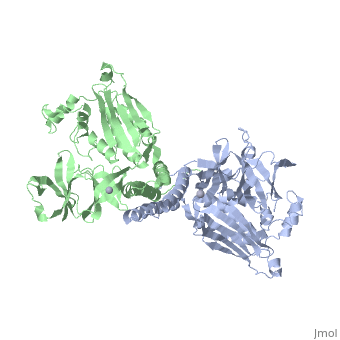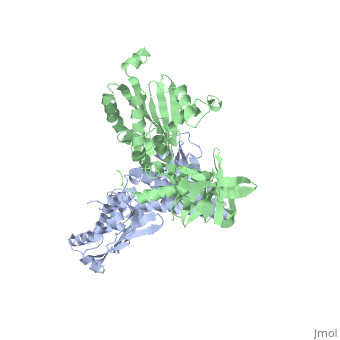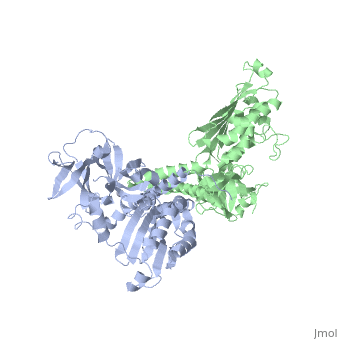
[[1b3q]] is a 2 chain structure with sequence from [ | <StructureSection load='1b3q' size='340' side='right'caption='[[1b3q]], [[Resolution|resolution]] 2.60Å' scene=''> | ||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1b3q]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Thermotoga_maritima Thermotoga maritima]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1B3Q OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1B3Q FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.6Å</td></tr> | |||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=HG:MERCURY+(II)+ION'>HG</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1b3q FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1b3q OCA], [https://pdbe.org/1b3q PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1b3q RCSB], [https://www.ebi.ac.uk/pdbsum/1b3q PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1b3q ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/CHEA_THEMA CHEA_THEMA] Involved in the transmission of sensory signals from the chemoreceptors to the flagellar motors. CheA is autophosphorylated; it can transfer its phosphate group to either CheB or CheY (By similarity). | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/b3/1b3q_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1b3q ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Histidine kinases allow bacteria, plants, and fungi to sense and respond to their environment. The 2.6 A resolution crystal structure of Thermotoga maritima CheA (290-671) histidine kinase reveals a dimer where the functions of dimerization, ATP binding, and regulation are segregated into domains. The kinase domain is unlike Ser/Thr/Tyr kinases but resembles two ATPases, Gyrase B and Hsp90. Structural analogies within this superfamily suggest that the P1 domain of CheA provides the nucleophilic histidine and activating glutamate for phosphotransfer. The regulatory domain, which binds the homologous receptor-coupling protein CheW, topologically resembles two SH3 domains and provides different protein recognition surfaces at each end. The dimerization domain forms a central four-helix bundle about which the kinase and regulatory domains pivot on conserved hinges to modulate transphosphorylation. Different subunit conformations suggest that relative domain motions link receptor response to kinase activity. | |||
Structure of CheA, a signal-transducing histidine kinase.,Bilwes AM, Alex LA, Crane BR, Simon MI Cell. 1999 Jan 8;96(1):131-41. PMID:9989504<ref>PMID:9989504</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 1b3q" style="background-color:#fffaf0;"></div> | |||
==See Also== | ==See Also== | ||
*[[Chemotaxis protein|Chemotaxis protein]] | *[[Chemotaxis protein 3D structures|Chemotaxis protein 3D structures]] | ||
== References == | |||
== | <references/> | ||
< | __TOC__ | ||
</StructureSection> | |||
[[Category: Large Structures]] | |||
[[Category: Thermotoga maritima]] | [[Category: Thermotoga maritima]] | ||
[[Category: Alex | [[Category: Alex LA]] | ||
[[Category: Bilwes | [[Category: Bilwes AM]] | ||
[[Category: Crane | [[Category: Crane BR]] | ||
[[Category: Simon | [[Category: Simon MI]] | ||
Latest revision as of 02:19, 28 December 2023
CRYSTAL STRUCTURE OF CHEA-289, A SIGNAL TRANSDUCING HISTIDINE KINASECRYSTAL STRUCTURE OF CHEA-289, A SIGNAL TRANSDUCING HISTIDINE KINASE
Structural highlights
FunctionCHEA_THEMA Involved in the transmission of sensory signals from the chemoreceptors to the flagellar motors. CheA is autophosphorylated; it can transfer its phosphate group to either CheB or CheY (By similarity). Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedHistidine kinases allow bacteria, plants, and fungi to sense and respond to their environment. The 2.6 A resolution crystal structure of Thermotoga maritima CheA (290-671) histidine kinase reveals a dimer where the functions of dimerization, ATP binding, and regulation are segregated into domains. The kinase domain is unlike Ser/Thr/Tyr kinases but resembles two ATPases, Gyrase B and Hsp90. Structural analogies within this superfamily suggest that the P1 domain of CheA provides the nucleophilic histidine and activating glutamate for phosphotransfer. The regulatory domain, which binds the homologous receptor-coupling protein CheW, topologically resembles two SH3 domains and provides different protein recognition surfaces at each end. The dimerization domain forms a central four-helix bundle about which the kinase and regulatory domains pivot on conserved hinges to modulate transphosphorylation. Different subunit conformations suggest that relative domain motions link receptor response to kinase activity. Structure of CheA, a signal-transducing histidine kinase.,Bilwes AM, Alex LA, Crane BR, Simon MI Cell. 1999 Jan 8;96(1):131-41. PMID:9989504[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences |
| ||||||||||||||||||