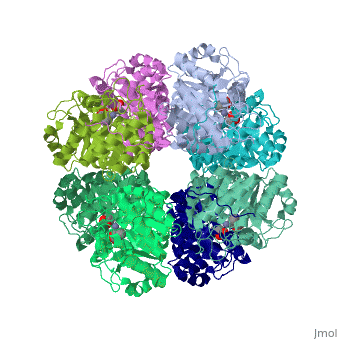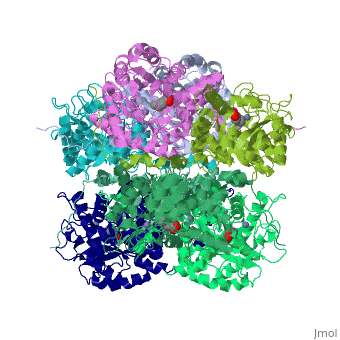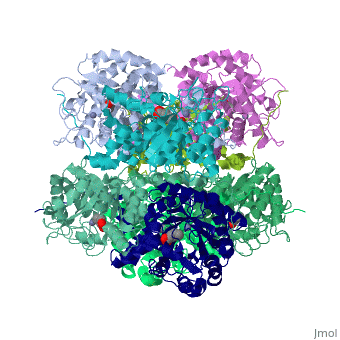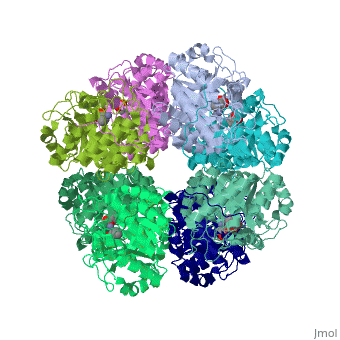1h7n: Difference between revisions
No edit summary |
No edit summary |
||
| (11 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
< | ==SCHIFF-BASE COMPLEX OF YEAST 5-AMINOLAEVULINIC ACID DEHYDRATASE WITH LAEVULINIC ACID AT 1.6 A RESOLUTION== | ||
<StructureSection load='1h7n' size='340' side='right'caption='[[1h7n]], [[Resolution|resolution]] 1.60Å' scene=''> | |||
You may | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1h7n]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Saccharomyces_cerevisiae Saccharomyces cerevisiae]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1H7N OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1H7N FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.6Å</td></tr> | |||
-- | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=SHF:LAEVULINIC+ACID'>SHF</scene>, <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1h7n FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1h7n OCA], [https://pdbe.org/1h7n PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1h7n RCSB], [https://www.ebi.ac.uk/pdbsum/1h7n PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1h7n ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/HEM2_YEAST HEM2_YEAST] Catalyzes an early step in the biosynthesis of tetrapyrroles. Binds two molecules of 5-aminolevulinate per subunit, each at a distinct site, and catalyzes their condensation to form porphobilinogen. | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/h7/1h7n_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1h7n ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
The structures of 5-aminolaevulinic acid dehydratase (ALAD) complexed with substrate (5-aminolaevulinic acid) and three inhibitors: laevulinic acid, succinylacetone and 4-keto-5-aminolaevulinic acid, have been solved at high resolution. The ligands all bind by forming a covalent link with Lys263 at the active site. The structures define the interactions made by one of the two substrate moieties that bind to the enzyme during catalysis. All of the inhibitors induce a significant ordering of the flap covering the active site. Succinylacetone appears to be unique by inducing a number of conformational changes in loops covering the active site, which may be important for understanding the co-operative properties of ALAD enzymes. Succinylacetone is produced in large amounts by patients suffering from the hereditary disease type I tyrosinaemia and its potent inhibition of ALAD also has implications for the pathology of this disease. The most intriguing result is that obtained with 4-keto-5-amino-hexanoic acid, which seems to form a stable carbinolamine intermediate with Lys263. It appears that we have defined the structure of an intermediate of Schiff base formation that the substrate forms upon binding to the P-site of the enzyme. | |||
The x-ray structure of yeast 5-aminolaevulinic acid dehydratase complexed with substrate and three inhibitors.,Erskine PT, Newbold R, Brindley AA, Wood SP, Shoolingin-Jordan PM, Warren MJ, Cooper JB J Mol Biol. 2001 Sep 7;312(1):133-41. PMID:11545591<ref>PMID:11545591</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 1h7n" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Porphobilinogen synthase|Porphobilinogen synthase]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
== | [[Category: Large Structures]] | ||
== | |||
[[Category: | |||
[[Category: Saccharomyces cerevisiae]] | [[Category: Saccharomyces cerevisiae]] | ||
[[Category: Brindley AA]] | |||
[[Category: Brindley | [[Category: Cooper JB]] | ||
[[Category: Cooper | [[Category: Erskine PT]] | ||
[[Category: Erskine | [[Category: Newbold R]] | ||
[[Category: Newbold | [[Category: Shoolingin-Jordan PM]] | ||
[[Category: Shoolingin-Jordan | [[Category: Warren MJ]] | ||
[[Category: Warren | [[Category: Wood SP]] | ||
[[Category: Wood | |||
Latest revision as of 15:19, 13 December 2023
SCHIFF-BASE COMPLEX OF YEAST 5-AMINOLAEVULINIC ACID DEHYDRATASE WITH LAEVULINIC ACID AT 1.6 A RESOLUTIONSCHIFF-BASE COMPLEX OF YEAST 5-AMINOLAEVULINIC ACID DEHYDRATASE WITH LAEVULINIC ACID AT 1.6 A RESOLUTION
Structural highlights
FunctionHEM2_YEAST Catalyzes an early step in the biosynthesis of tetrapyrroles. Binds two molecules of 5-aminolevulinate per subunit, each at a distinct site, and catalyzes their condensation to form porphobilinogen. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe structures of 5-aminolaevulinic acid dehydratase (ALAD) complexed with substrate (5-aminolaevulinic acid) and three inhibitors: laevulinic acid, succinylacetone and 4-keto-5-aminolaevulinic acid, have been solved at high resolution. The ligands all bind by forming a covalent link with Lys263 at the active site. The structures define the interactions made by one of the two substrate moieties that bind to the enzyme during catalysis. All of the inhibitors induce a significant ordering of the flap covering the active site. Succinylacetone appears to be unique by inducing a number of conformational changes in loops covering the active site, which may be important for understanding the co-operative properties of ALAD enzymes. Succinylacetone is produced in large amounts by patients suffering from the hereditary disease type I tyrosinaemia and its potent inhibition of ALAD also has implications for the pathology of this disease. The most intriguing result is that obtained with 4-keto-5-amino-hexanoic acid, which seems to form a stable carbinolamine intermediate with Lys263. It appears that we have defined the structure of an intermediate of Schiff base formation that the substrate forms upon binding to the P-site of the enzyme. The x-ray structure of yeast 5-aminolaevulinic acid dehydratase complexed with substrate and three inhibitors.,Erskine PT, Newbold R, Brindley AA, Wood SP, Shoolingin-Jordan PM, Warren MJ, Cooper JB J Mol Biol. 2001 Sep 7;312(1):133-41. PMID:11545591[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||