1e8o: Difference between revisions
No edit summary |
No edit summary |
||
| (11 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
< | ==Core of the Alu domain of the mammalian SRP== | ||
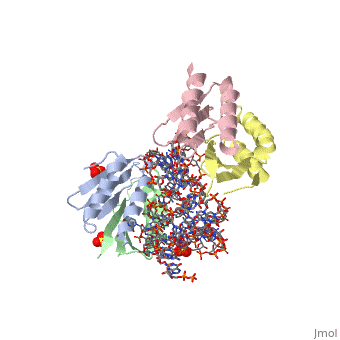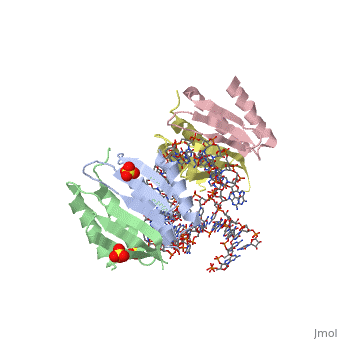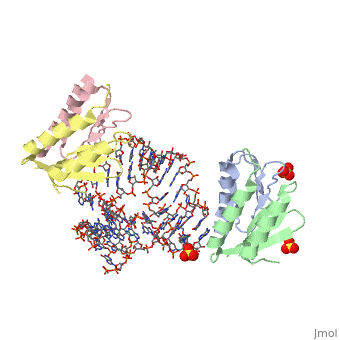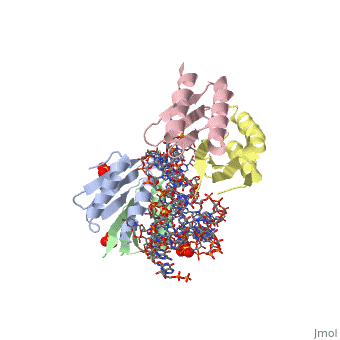
<StructureSection load='1e8o' size='340' side='right'caption='[[1e8o]], [[Resolution|resolution]] 3.20Å' scene=''> | |||
You may | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1e8o]] is a 5 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1E8O OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1E8O FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.2Å</td></tr> | |||
- | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=GDP:GUANOSINE-5-DIPHOSPHATE'>GDP</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1e8o FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1e8o OCA], [https://pdbe.org/1e8o PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1e8o RCSB], [https://www.ebi.ac.uk/pdbsum/1e8o PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1e8o ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/SRP09_HUMAN SRP09_HUMAN] Signal-recognition-particle assembly has a crucial role in targeting secretory proteins to the rough endoplasmic reticulum membrane. SRP9 together with SRP14 and the Alu portion of the SRP RNA, constitutes the elongation arrest domain of SRP. The complex of SRP9 and SRP14 is required for SRP RNA binding. | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/e8/1e8o_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1e8o ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
The Alu domain of the mammalian signal recognition particle (SRP) comprises the heterodimer of proteins SRP9 and SRP14 bound to the 5' and 3' terminal sequences of SRP RNA. It retards the ribosomal elongation of signal-peptide-containing proteins before their engagement with the translocation machinery in the endoplasmic reticulum. Here we report two crystal structures of the heterodimer SRP9/14 bound either to the 5' domain or to a construct containing both 5' and 3' domains. We present a model of the complete Alu domain that is consistent with extensive biochemical data. SRP9/14 binds strongly to the conserved core of the 5' domain, which forms a U-turn connecting two helical stacks. Reversible docking of the more weakly bound 3' domain might be functionally important in the mechanism of translational regulation. The Alu domain structure is probably conserved in other cytoplasmic ribonucleoprotein particles and retroposition intermediates containing SRP9/14-bound RNAs transcribed from Alu repeats or related elements in genomic DNA. | |||
Structure and assembly of the Alu domain of the mammalian signal recognition particle.,Weichenrieder O, Wild K, Strub K, Cusack S Nature. 2000 Nov 9;408(6809):167-73. PMID:11089964<ref>PMID:11089964</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 1e8o" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Signal recognition particle 3D structures|Signal recognition particle 3D structures]] | |||
*[[Signal recognition particle protein|Signal recognition particle protein]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
== | </StructureSection> | ||
== | |||
< | |||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Cusack | [[Category: Large Structures]] | ||
[[Category: Strub | [[Category: Cusack S]] | ||
[[Category: Weichenrieder | [[Category: Strub K]] | ||
[[Category: Wild | [[Category: Weichenrieder O]] | ||
[[Category: Wild K]] | |||
Latest revision as of 14:56, 13 December 2023
Core of the Alu domain of the mammalian SRPCore of the Alu domain of the mammalian SRP
Structural highlights
FunctionSRP09_HUMAN Signal-recognition-particle assembly has a crucial role in targeting secretory proteins to the rough endoplasmic reticulum membrane. SRP9 together with SRP14 and the Alu portion of the SRP RNA, constitutes the elongation arrest domain of SRP. The complex of SRP9 and SRP14 is required for SRP RNA binding. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe Alu domain of the mammalian signal recognition particle (SRP) comprises the heterodimer of proteins SRP9 and SRP14 bound to the 5' and 3' terminal sequences of SRP RNA. It retards the ribosomal elongation of signal-peptide-containing proteins before their engagement with the translocation machinery in the endoplasmic reticulum. Here we report two crystal structures of the heterodimer SRP9/14 bound either to the 5' domain or to a construct containing both 5' and 3' domains. We present a model of the complete Alu domain that is consistent with extensive biochemical data. SRP9/14 binds strongly to the conserved core of the 5' domain, which forms a U-turn connecting two helical stacks. Reversible docking of the more weakly bound 3' domain might be functionally important in the mechanism of translational regulation. The Alu domain structure is probably conserved in other cytoplasmic ribonucleoprotein particles and retroposition intermediates containing SRP9/14-bound RNAs transcribed from Alu repeats or related elements in genomic DNA. Structure and assembly of the Alu domain of the mammalian signal recognition particle.,Weichenrieder O, Wild K, Strub K, Cusack S Nature. 2000 Nov 9;408(6809):167-73. PMID:11089964[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||