Stathmin: Difference between revisions
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
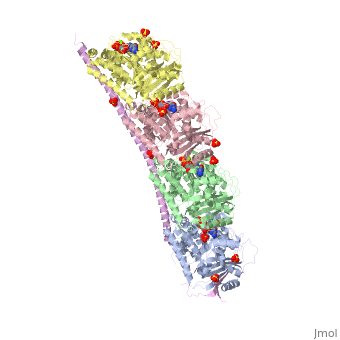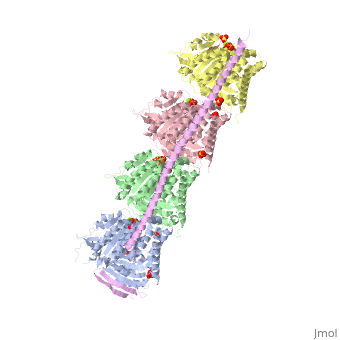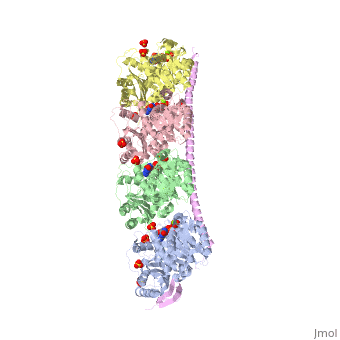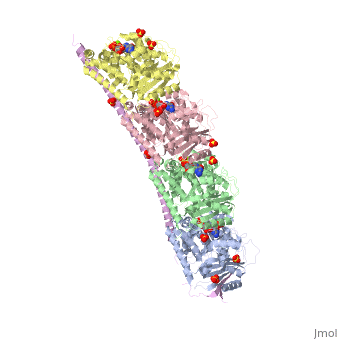
<StructureSection load='3ryf' size='340' side='right' caption='Rat stathmin SLD domain (magenta) complex with tubulin α chain (grey, pink), tubulin β chain (green, yellow), GTP, sulfate and Mg+2 ions (PDB code [[3ryf]])' scene=''> | <StructureSection load='3ryf' size='340' side='right' caption='Rat stathmin SLD domain (magenta) complex with tubulin α chain (grey, pink), tubulin β chain (green, yellow), GTP, sulfate and Mg+2 ions (PDB code [[3ryf]])' scene=''> | ||
== Function == | == Function == | ||
'''Stathmin-4''' (STM-4) regulates microtubules dynamics. Microtubules undergo continuous assembly and disassembly in the cell’s cytoskeleton. STM-4 binds to tubulin and prevents the latter from polymerization thus preventing microtubule assembly. Phosphorylation of STM-4 weakens the binding of STM-4 to tubulin enabling the microtubule assembly needed for the formation of mitotic spindle. Thus, STM-4 is an oncoprotein. STM-4 contains an SLD (Stathmin-Like Domain) domain of 149 residues which binds the tubulin dimer<ref>PMID:15216892</ref>. | '''Stathmin-4''' (STM-4) or '''stathmin-like protein B3''' regulates microtubules dynamics. Microtubules undergo continuous assembly and disassembly in the cell’s cytoskeleton. STM-4 binds to tubulin and prevents the latter from polymerization thus preventing microtubule assembly. Phosphorylation of STM-4 weakens the binding of STM-4 to tubulin enabling the microtubule assembly needed for the formation of mitotic spindle. Thus, STM-4 is an oncoprotein. STM-4 contains an SLD (Stathmin-Like Domain) domain of 149 residues which binds the tubulin dimer<ref>PMID:15216892</ref>. | ||
== Disease == | == Disease == | ||
| Line 11: | Line 11: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Latest revision as of 14:02, 4 December 2023
FunctionStathmin-4 (STM-4) or stathmin-like protein B3 regulates microtubules dynamics. Microtubules undergo continuous assembly and disassembly in the cell’s cytoskeleton. STM-4 binds to tubulin and prevents the latter from polymerization thus preventing microtubule assembly. Phosphorylation of STM-4 weakens the binding of STM-4 to tubulin enabling the microtubule assembly needed for the formation of mitotic spindle. Thus, STM-4 is an oncoprotein. STM-4 contains an SLD (Stathmin-Like Domain) domain of 149 residues which binds the tubulin dimer[1]. DiseaseDecreased levels of STM-4 are found in brains of adults with Down syndrome and Alzheimer disease[2]. 3D Structures of stathmin-4
|
| ||||||||||
ReferencesReferences
- ↑ Curmi PA, Gavet O, Charbaut E, Ozon S, Lachkar-Colmerauer S, Manceau V, Siavoshian S, Maucuer A, Sobel A. Stathmin and its phosphoprotein family: general properties, biochemical and functional interaction with tubulin. Cell Struct Funct. 1999 Oct;24(5):345-57. PMID:15216892
- ↑ Cheon MS, Fountoulakis M, Cairns NJ, Dierssen M, Herkner K, Lubec G. Decreased protein levels of stathmin in adult brains with Down syndrome and Alzheimer's disease. J Neural Transm Suppl. 2001;(61):281-8. PMID:11771751