2djh: Difference between revisions
No edit summary |
No edit summary |
||
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
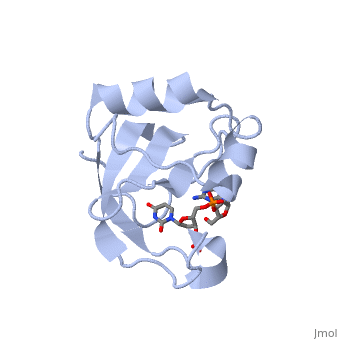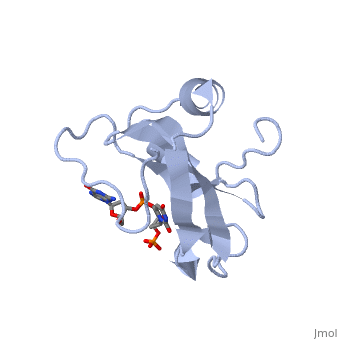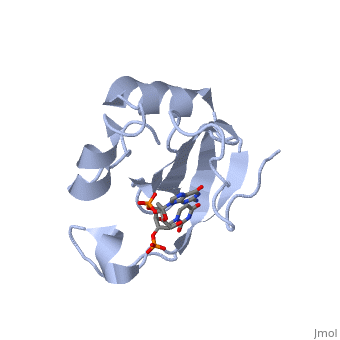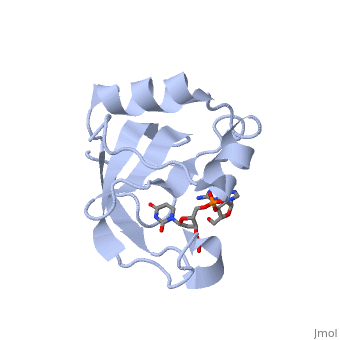
==Crystal structure of the carboxy-terminal ribonuclease domain of Colicin E5== | |||
<StructureSection load='2djh' size='340' side='right'caption='[[2djh]], [[Resolution|resolution]] 1.90Å' scene=''> | |||
| | == Structural highlights == | ||
<table><tr><td colspan='2'>[[2djh]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2DJH OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2DJH FirstGlance]. <br> | |||
| | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.9Å</td></tr> | ||
| | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=3PD:2-AMINO-9-(2-DEOXY-3-O-PHOSPHONOPENTOFURANOSYL)-1,9-DIHYDRO-6H-PURIN-6-ONE'>3PD</scene>, <scene name='pdbligand=UM3:2-DEOXYURIDINE+3-MONOPHOSPHATE'>UM3</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2djh FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2djh OCA], [https://pdbe.org/2djh PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2djh RCSB], [https://www.ebi.ac.uk/pdbsum/2djh PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2djh ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/CEA5_ECOLX CEA5_ECOLX] Colicins are polypeptide toxins produced by and active against E.coli and closely related bacteria. This colicin is an endonuclease. | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/dj/2djh_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2djh ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Colicin E5--a tRNase toxin--specifically cleaves QUN (Q: queuosine) anticodons of the Escherichia coli tRNAs for Tyr, His, Asn and Asp. Here, we report the crystal structure of the C-terminal ribonuclease domain (CRD) of E5 complexed with a substrate analog, namely, dGpdUp, at a resolution of 1.9 A. Thisstructure is the first to reveal the substrate recognition mechanism of sequence-specific ribonucleases. E5-CRD realized the strict recognition for both the guanine and uracil bases of dGpdUp forming Watson-Crick-type hydrogen bonds and ring stacking interactions, thus mimicking the codons of mRNAs to bind to tRNA anticodons. The docking model of E5-CRD with tRNA also suggests its substrate preference for tRNA over ssRNA. In addition, the structure of E5-CRD/dGpdUp along with the mutational analysis suggests that Arg33 may play an important role in the catalytic activity, and Lys25/Lys60 may also be involved without His in E5-CRD. Finally, the comparison of the structures of E5-CRD/dGpdUp and E5-CRD/ImmE5 (an inhibitor protein) complexes suggests that the binding mode of E5-CRD and ImmE5 mimics that of mRNA and tRNA; this may represent the evolutionary pathway of these proteins from the RNA-RNA interaction through the RNA-protein interaction of tRNA/E5-CRD. | |||
Structural basis for sequence-dependent recognition of colicin E5 tRNase by mimicking the mRNA-tRNA interaction.,Yajima S, Inoue S, Ogawa T, Nonaka T, Ohsawa K, Masaki H Nucleic Acids Res. 2006;34(21):6074-82. Epub 2006 Nov 11. PMID:17099236<ref>PMID:17099236</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 2djh" style="background-color:#fffaf0;"></div> | |||
== | ==See Also== | ||
Colicin | *[[Colicin 3D structures|Colicin 3D structures]] | ||
== References == | |||
== | <references/> | ||
__TOC__ | |||
</StructureSection> | |||
[[Category: Escherichia coli]] | [[Category: Escherichia coli]] | ||
[[Category: | [[Category: Large Structures]] | ||
[[Category: Inoue | [[Category: Inoue S]] | ||
[[Category: Masaki | [[Category: Masaki H]] | ||
[[Category: Nonaka | [[Category: Nonaka T]] | ||
[[Category: Ogawa | [[Category: Ogawa T]] | ||
[[Category: Ohsawa | [[Category: Ohsawa K]] | ||
[[Category: Yajima | [[Category: Yajima S]] | ||
Latest revision as of 11:27, 25 October 2023
Crystal structure of the carboxy-terminal ribonuclease domain of Colicin E5Crystal structure of the carboxy-terminal ribonuclease domain of Colicin E5
Structural highlights
FunctionCEA5_ECOLX Colicins are polypeptide toxins produced by and active against E.coli and closely related bacteria. This colicin is an endonuclease. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedColicin E5--a tRNase toxin--specifically cleaves QUN (Q: queuosine) anticodons of the Escherichia coli tRNAs for Tyr, His, Asn and Asp. Here, we report the crystal structure of the C-terminal ribonuclease domain (CRD) of E5 complexed with a substrate analog, namely, dGpdUp, at a resolution of 1.9 A. Thisstructure is the first to reveal the substrate recognition mechanism of sequence-specific ribonucleases. E5-CRD realized the strict recognition for both the guanine and uracil bases of dGpdUp forming Watson-Crick-type hydrogen bonds and ring stacking interactions, thus mimicking the codons of mRNAs to bind to tRNA anticodons. The docking model of E5-CRD with tRNA also suggests its substrate preference for tRNA over ssRNA. In addition, the structure of E5-CRD/dGpdUp along with the mutational analysis suggests that Arg33 may play an important role in the catalytic activity, and Lys25/Lys60 may also be involved without His in E5-CRD. Finally, the comparison of the structures of E5-CRD/dGpdUp and E5-CRD/ImmE5 (an inhibitor protein) complexes suggests that the binding mode of E5-CRD and ImmE5 mimics that of mRNA and tRNA; this may represent the evolutionary pathway of these proteins from the RNA-RNA interaction through the RNA-protein interaction of tRNA/E5-CRD. Structural basis for sequence-dependent recognition of colicin E5 tRNase by mimicking the mRNA-tRNA interaction.,Yajima S, Inoue S, Ogawa T, Nonaka T, Ohsawa K, Masaki H Nucleic Acids Res. 2006;34(21):6074-82. Epub 2006 Nov 11. PMID:17099236[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||