3pih: Difference between revisions
No edit summary |
No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
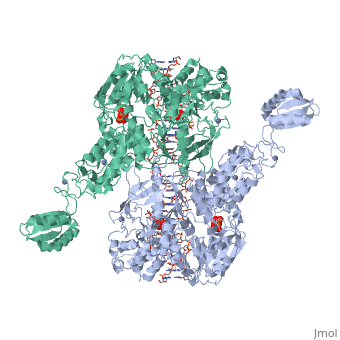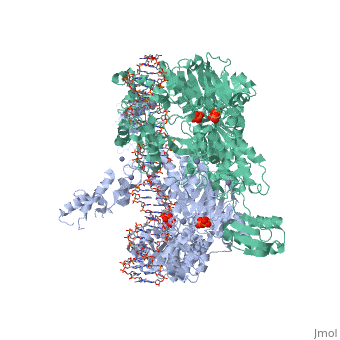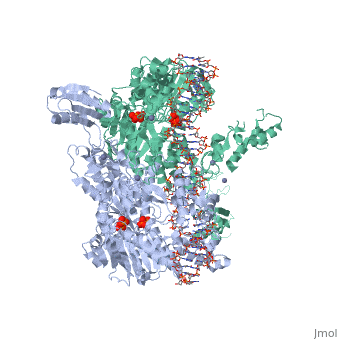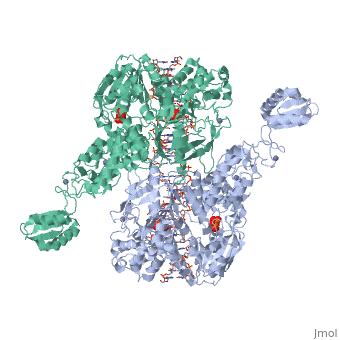
< | ==T. maritima UvrA in complex with fluorescein-modified DNA== | ||
<StructureSection load='3pih' size='340' side='right'caption='[[3pih]], [[Resolution|resolution]] 2.90Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[3pih]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Thermotoga_maritima Thermotoga maritima]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3PIH OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3PIH FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.9Å</td></tr> | |||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=PPV:PYROPHOSPHATE'>PPV</scene>, <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3pih FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3pih OCA], [https://pdbe.org/3pih PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3pih RCSB], [https://www.ebi.ac.uk/pdbsum/3pih PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3pih ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/UVRA_THEMA UVRA_THEMA] The UvrABC repair system catalyzes the recognition and processing of DNA lesions. UvrA is an ATPase and a DNA-binding protein. A damage recognition complex composed of 2 UvrA and 2 UvrB subunits scans DNA for abnormalities. When the presence of a lesion has been verified by UvrB, the UvrA molecules dissociate (By similarity). | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
One of the primary pathways for removal of DNA damage is nucleotide excision repair (NER). In bacteria, the UvrA protein is the component of NER that locates the lesion. A notable feature of NER is its ability to act on many DNA modifications that vary in chemical structure. So far, the mechanism underlying this broad specificity has been unclear. Here, we report the first crystal structure of a UvrA protein in complex with a chemically modified oligonucleotide. The structure shows that the UvrA dimer does not contact the site of lesion directly, but rather binds the DNA regions on both sides of the modification. The DNA region harboring the modification is deformed, with the double helix bent and unwound. UvrA uses damage-induced deformations of the DNA and a less rigid structure of the modified double helix for indirect readout of the lesion. | |||
Structure of UvrA nucleotide excision repair protein in complex with modified DNA.,Jaciuk M, Nowak E, Skowronek K, Tanska A, Nowotny M Nat Struct Mol Biol. 2011 Feb;18(2):191-7. Epub 2011 Jan 16. PMID:21240268<ref>PMID:21240268</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 3pih" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[UvrABC|UvrABC]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
== | [[Category: Large Structures]] | ||
[[ | |||
== | |||
< | |||
[[Category: Thermotoga maritima]] | [[Category: Thermotoga maritima]] | ||
[[Category: Jaciuk | [[Category: Jaciuk M]] | ||
[[Category: Nowak | [[Category: Nowak E]] | ||
[[Category: Nowotny | [[Category: Nowotny M]] | ||
Latest revision as of 12:55, 6 September 2023
T. maritima UvrA in complex with fluorescein-modified DNAT. maritima UvrA in complex with fluorescein-modified DNA
Structural highlights
FunctionUVRA_THEMA The UvrABC repair system catalyzes the recognition and processing of DNA lesions. UvrA is an ATPase and a DNA-binding protein. A damage recognition complex composed of 2 UvrA and 2 UvrB subunits scans DNA for abnormalities. When the presence of a lesion has been verified by UvrB, the UvrA molecules dissociate (By similarity). Publication Abstract from PubMedOne of the primary pathways for removal of DNA damage is nucleotide excision repair (NER). In bacteria, the UvrA protein is the component of NER that locates the lesion. A notable feature of NER is its ability to act on many DNA modifications that vary in chemical structure. So far, the mechanism underlying this broad specificity has been unclear. Here, we report the first crystal structure of a UvrA protein in complex with a chemically modified oligonucleotide. The structure shows that the UvrA dimer does not contact the site of lesion directly, but rather binds the DNA regions on both sides of the modification. The DNA region harboring the modification is deformed, with the double helix bent and unwound. UvrA uses damage-induced deformations of the DNA and a less rigid structure of the modified double helix for indirect readout of the lesion. Structure of UvrA nucleotide excision repair protein in complex with modified DNA.,Jaciuk M, Nowak E, Skowronek K, Tanska A, Nowotny M Nat Struct Mol Biol. 2011 Feb;18(2):191-7. Epub 2011 Jan 16. PMID:21240268[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||