2pzh: Difference between revisions
No edit summary |
No edit summary |
||
| (8 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
< | ==YbgC thioesterase (Hp0496) from Helicobacter pylori== | ||
<StructureSection load='2pzh' size='340' side='right'caption='[[2pzh]], [[Resolution|resolution]] 1.70Å' scene=''> | |||
You may | == Structural highlights == | ||
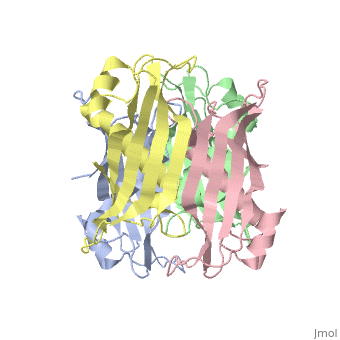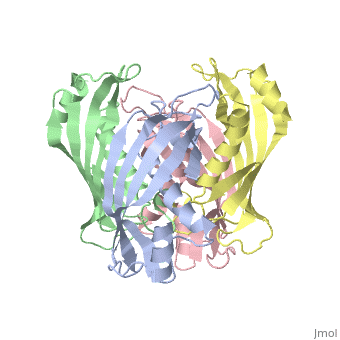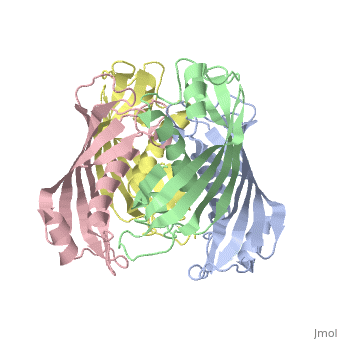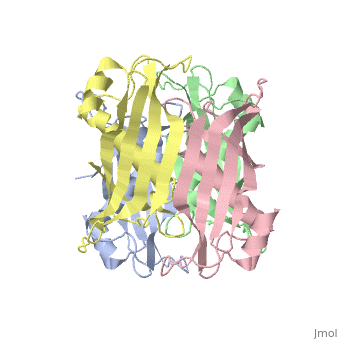
<table><tr><td colspan='2'>[[2pzh]] is a 4 chain structure with sequence from [https://en.wikipedia.org/wiki/Helicobacter_pylori_26695 Helicobacter pylori 26695]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2PZH OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2PZH FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.7Å</td></tr> | |||
-- | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2pzh FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2pzh OCA], [https://pdbe.org/2pzh PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2pzh RCSB], [https://www.ebi.ac.uk/pdbsum/2pzh PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2pzh ProSAT]</span></td></tr> | ||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/YBGC_HELPY YBGC_HELPY] Thioesterase that may be involved in phospholipid metabolism. Displays acyl-CoA thioesterase activity with lauroyl-CoA (C12:0), myristoyl-CoA (C14:0), palmitoyl-CoA (C16:0), stearoyl-CoA (C18:0) and benzoyl-CoA, catalyzing the hydrolysis of the thioester bond. Has low activity with butyryl-CoA and octanoyl-CoA.<ref>PMID:18338382</ref> | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/pz/2pzh_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2pzh ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
YbgC proteins are bacterial acyl-CoA thioesterases associated with the Tol-Pal system. This system is important for cell envelope integrity and is part of the cell division machinery. In E. coli, YbgC associates with the cell membrane and is part of a protein network involved in lipid biogenesis. In the human pathogen Helicobacter pylori, a putative homologue of YbgC, named HP0496, was found to interact with the cytotoxin CagA by two different studies. We have determined its crystal structure and characterized its enzymatic activity. The structure of HP0496 shows that it is a member of the hot-dog family of proteins, with a epsilongamma tetrameric arrangement. Finally, enzymatic assays performed with the purified protein showed that HP0496 is an acyl-CoA thioesterase that favors long-chain substrates. Proteins 2008. (c) 2008 Wiley-Liss, Inc. | |||
Structural and enzymatic characterization of HP0496, a YbgC thioesterase from Helicobacter pylori.,Angelini A, Cendron L, Goncalves S, Zanotti G, Terradot L Proteins. 2008 Mar 12;72(4):1212-1221. PMID:18338382<ref>PMID:18338382</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 2pzh" style="background-color:#fffaf0;"></div> | |||
==See Also== | |||
*[[Thioesterase 3D structures|Thioesterase 3D structures]] | |||
== References == | |||
<references/> | |||
__TOC__ | |||
</StructureSection> | |||
== | |||
== | |||
[[Category: Helicobacter pylori 26695]] | [[Category: Helicobacter pylori 26695]] | ||
[[Category: | [[Category: Large Structures]] | ||
[[Category: Angelini | [[Category: Angelini A]] | ||
[[Category: Cendron | [[Category: Cendron L]] | ||
[[Category: Goncalves | [[Category: Goncalves S]] | ||
[[Category: Terradot | [[Category: Terradot L]] | ||
[[Category: Zanotti | [[Category: Zanotti G]] | ||
Latest revision as of 14:13, 30 August 2023
YbgC thioesterase (Hp0496) from Helicobacter pyloriYbgC thioesterase (Hp0496) from Helicobacter pylori
Structural highlights
FunctionYBGC_HELPY Thioesterase that may be involved in phospholipid metabolism. Displays acyl-CoA thioesterase activity with lauroyl-CoA (C12:0), myristoyl-CoA (C14:0), palmitoyl-CoA (C16:0), stearoyl-CoA (C18:0) and benzoyl-CoA, catalyzing the hydrolysis of the thioester bond. Has low activity with butyryl-CoA and octanoyl-CoA.[1] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedYbgC proteins are bacterial acyl-CoA thioesterases associated with the Tol-Pal system. This system is important for cell envelope integrity and is part of the cell division machinery. In E. coli, YbgC associates with the cell membrane and is part of a protein network involved in lipid biogenesis. In the human pathogen Helicobacter pylori, a putative homologue of YbgC, named HP0496, was found to interact with the cytotoxin CagA by two different studies. We have determined its crystal structure and characterized its enzymatic activity. The structure of HP0496 shows that it is a member of the hot-dog family of proteins, with a epsilongamma tetrameric arrangement. Finally, enzymatic assays performed with the purified protein showed that HP0496 is an acyl-CoA thioesterase that favors long-chain substrates. Proteins 2008. (c) 2008 Wiley-Liss, Inc. Structural and enzymatic characterization of HP0496, a YbgC thioesterase from Helicobacter pylori.,Angelini A, Cendron L, Goncalves S, Zanotti G, Terradot L Proteins. 2008 Mar 12;72(4):1212-1221. PMID:18338382[2] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||